A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many pi electrons are in the excited state LUMO for buta-1,3-diene?
A) 0
B) 1
C) 2
D) 3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What type of cycloaddition reaction is shown below? ![What type of cycloaddition reaction is shown below? A) [2+2] B) [4+2] C) [4+4] D) [0+2]](https://d2lvgg3v3hfg70.cloudfront.net/TB7662/11eac43c_f775_1a2a_a9af_a506ca08166f_TB7662_00.jpg)
A) [2+2]
B) [4+2]
C) [4+4]
D) [0+2]
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many pi electrons are in the excited state HOMO for buta-1,3-diene?
A) 0
B) 1
C) 2
D) 3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many bonding molecular orbitals are present in 1,3,5-hexatriene?
A) 3
B) 4
C) 5
D) 6
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about electrocyclic ring closure is not true?
A) It is an intramolecular reaction.
B) It requires heat or light.
C) The cyclic product contains one more s bond and one fewer p bond than the reactants.
D) The cyclic product contains one more p bond and one fewer s bond than the reactants.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about the Claisen rearrangement is true?
A) The Claisen rearrangement occurs readily in a suprafacial pathway under photochemical conditions.
B) The Claisen rearrangement occurs readily in an antarafacial pathway under thermal conditions.
C) The Claisen rearrangement involves three electron pairs; two in p bonds and one in a s bond.
D) The Claisen rearrangement involves the rearrangement of an unsaturated ether to a b,g-unsaturated carbonyl compound.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major organic product of the following Claisen rearrangement? 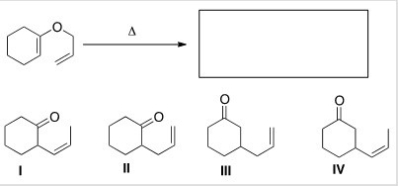
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about cycloaddition reactions is true?
A) Cycloaddition reactions occur only intermolecularly.
B) Cycloaddition reactions occur only intramolecularly.
C) Cycloaddition reactions form a cyclic product with two new p bonds.
D) Cycloaddition reactions can be intramolecular or intermolecular.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about molecular orbitals is true?
A) The number of molecular orbitals formed is different from the number of atomic orbitals used.
B) The number of molecular orbitals formed is equal to the number of atomic orbitals used.
C) The number of molecular orbitals formed is equal to twice the number of atomic orbitals used.
D) The number of molecular orbitals formed is equal to half the number of atomic orbitals used.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about sigmatropic rearrangements and orbital symmetry is not true?
A) Reactions involving six atoms or fewer must take place by suprafacial pathways.
B) In a suprafacial rearrangement,the new s bond forms on the opposite side of the p system as the broken s bond.
C) In an antarafacial rearrangement,the new s bond forms on the opposite side of the p system as the broken s bond.
D) In a suprafacial rearrangement,the new s bond forms on the same side of the p system as the broken s bond.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not a type of pericyclic reactions?
A) cycloadditions
B) nucleophilic reactions
C) electrocyclic reactions
D) sigmatropic rearrangements
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many bonding molecular orbitals are present in 1,3,5,7,9-decapentaene?
A) 4
B) 5
C) 10
D) 12
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many pi electrons are in the ground state HOMO for hexa-1,3,5-triene?
A) 0
B) 2
C) 4
D) 6
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about a thermal reaction involving an even number of electron pairs is true?
A) A thermal reaction involving an even number of electron pairs is conrotatory or antarafacial.
B) A thermal reaction involving an even number of electron pairs is disrotatory or suprafacial.
C) A thermal reaction involving an even number of electron pairs is conrotatory or suprafacial.
D) A thermal reaction involving an even number of electron pairs is disrotatory or antarafacial.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many pi electrons are in the ground state HOMO for buta-1,3-diene?
A) 0
B) 2
C) 4
D) 6
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about porbitals is true?
A) p orbitals are formed by the linear combination of two sp3 orbitals.
B) p orbitals are formed by the linear combination of two sp2 orbitals.
C) p orbitals are formed by the linear combination of two sp orbitals.
D) p orbitals are formed by the linear combination of two p orbitals.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about sigmatropic reactions is true?
A) The reactants contain one more p bond than the product.
B) The product contains one more p bond than the reactant.
C) A p bond is broken in the reactant.
D) A s bond is broken in the reactant.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a type of pericyclic reaction?
A) electrocyclic reactions
B) acyclic reactions
C) cycloelimination reactions
D) electrophilic reactions
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the organic product of the following electrocyclic reaction? 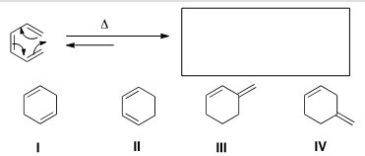
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 62
Related Exams