A) 37.5 K
B) 75 K
C) 106 K
D) 292 K
E) 2400. K
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of methane gas, CH4(g) , occupies a volume of 60.3 L at a pressure of 469 torr and a temperature of 29.3°C. What would be its temperature at a pressure of 243 torr and volume of 60.3 L?
A) -116.5°C
B) 15.2°C
C) 15.5°C
D) 57.7°C
E) 310.6°C
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the pressure in a 7.50-L flask if 0.15 mol of carbon dioxide is added to 0.33 mol of oxygen? The temperature of the mixture is 48.0°C.
A) 0.252 atm
B) 0.592 atm
C) 1.69 atm
D) 3.96 atm
E) 4.80 atm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the van der Waals equation for real gases to calculate the pressure exerted by 1.00 mole of ammonia at 27°C in a 750-mL container. (a = 4.17 L2·atm/mol2, b = 0.0371 L/mol)
A) 23.2 atm
B) 27.1 atm
C) 32.8 atm
D) 42.0 atm
E) 32.8 torr
Correct Answer

verified
Correct Answer
verified
Multiple Choice
"The volume of an ideal gas is directly proportional to its absolute temperature at constant pressure and number of moles" is a statement of ________________ Law.
A) Charles's
B) Boyle's
C) Amontons's
D) Avogadro's
E) Dalton's
Correct Answer

verified
Correct Answer
verified
True/False
For real gases, PV < nRT, always.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A gas mixture consists of equal masses of methane (molecular weight 16.0) and argon (atomic weight 40.0) . If the partial pressure of argon is 200. torr, what is the pressure of methane, in torr?
A) 80.0 torr
B) 200. torr
C) 256 torr
D) 500. torr
E) 556 torr
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of oxygen gas has its absolute temperature halved while the pressure of the gas remained constant. If the initial volume is 400 mL, what is the final volume?
A) 20 mL
B) 133 mL
C) 200 mL
D) 400 mL
E) 800 mL
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Arrange the following gases in order of increasing rate of effusion. C2H6 Ar HCl PH3
A) Ar < HCl < PH3 < C2H6
B) C2H6 < PH3 < HCl < Ar
C) Ar < PH3 < C2H6 < HCl
D) C2H6 < HCl < PH3 < Ar
E) Ar < PH3< HCl < C2H6
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The temperature of the carbon dioxide atmosphere near the surface of Venus is 475°C. Calculate the average kinetic energy per mole of carbon dioxide molecules on Venus.
A) 2520 J/mol
B) 4150 J/mol
C) 5920 J/mol
D) 9330 J/mol
E) 5920 kJ/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The pressure of sulfur dioxide in a container is 159 kPa. What is this pressure in atmospheres?
A) 0.209 atm
B) 0.637 atm
C) 1.57 atm
D) 21.2 atm
E) 15900 atm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Lithium oxide is an effective absorber of carbon dioxide and can be used to purify air in confined areas such as space vehicles. What volume of carbon dioxide can be absorbed by 1.00 kg of lithium oxide at 25°C and 1.00 atm? Li2O(aq) + CO2(g) → Li2CO3(s)
A) 687 mL
B) 819 mL
C) 687 L
D) 819 L
E) 22.4 L
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The ideal gas law tends to become inaccurate when
A) the pressure is lowered and molecular interactions become significant.
B) the pressure is raised and the temperature is lowered.
C) the temperature is raised above the temperature of STP.
D) large gas samples are involved.
E) the volume expands beyond the standard molar volume.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Freon-12, CF2Cl2, which has been widely used in air conditioning systems, is considered a threat to the ozone layer in the stratosphere. Calculate the root-mean-square velocity of Freon-12 molecules in the lower stratosphere where the temperature is -65°C.
A) 20 m/s
B) 120 m/s
C) 210 m/s
D) 260 m/s
E) 4.4 × 104 m/s
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 750-mL sample of hydrogen exerts a pressure of 822 torr at 325 K. What pressure does it exert if the temperature is raised to 475 K at constant volume?
A) 188 torr
B) 562 torr
C) 1.11 × 103 torr
D) 1.20 × 103 torr
E) 1.90 × 103 torr
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Convert a gas pressure of 485 cmHg to atmospheres.
A) 0.64 atm
B) 33.0 atm
C) 6.38 atm
D) 5.50 atm
E) 6.46 atm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the density of carbon dioxide gas at -25.2°C and 98.0 kPa?
A) 0.232 g/L
B) 0.279 g/L
C) 0.994 g/L
D) 1.74 g/L
E) 2.09 g/L
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the lines on the figure below is the best representation of the relationship between the volume and the number of moles of a gas, measured at constant temperature and pressure? 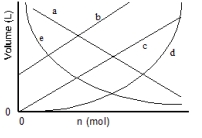
A) a
B) b
C) c
D) d
E) e
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the lines on the figure below is the best representation of the relationship between the volume of a gas and its absolute temperature, other factors remaining constant? 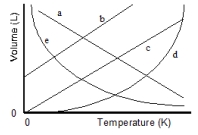
A) a
B) b
C) c
D) d
E) e
Correct Answer

verified
Correct Answer
verified
Multiple Choice
"The pressure of an ideal gas is inversely proportional to its volume at constant temperature and number of moles" is a statement of __________________ Law.
A) Charles's
B) Boyle's
C) Amontons's
D) Avogadro's
E) Gay-Lussac's
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 97
Related Exams