Kc is 1.67 × 1020 mol3 L-3 at 25 °C for the formation of iron(III) oxalate complex ion: Fe3+(aq) + 3C2O42-(aq) ⇌ [Fe(C2O4) 3]3-(aq) If 0. 0200 mol L-1 Fe3+ is initially mixed with 1.00 mol L-1 oxalate ion, what is the concentration of Fe3+ ion at equilibrium?
A) 1.44 × 10-22 mol L-1
B) 0.0100 mol L-1
C) 8.35 × 1019 mol L-1
D) 6.94 × 1021 mol L-1
F) A) and D)
Correct Answer

verified
Correct Answer
verified
Dinitrogen tetroxide partially decomposes according to the following equilibrium: 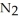
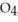 (g) ⇌
(g) ⇌ 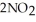 (g) A 1.000 L flask is charged with 6.00 × 10-2 mol of
(g) A 1.000 L flask is charged with 6.00 × 10-2 mol of 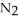
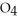 . At equilibrium, 3.21 × 10-2 mol of
. At equilibrium, 3.21 × 10-2 mol of 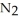
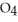 remains.
remains. 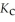 for this reaction is ________.
for this reaction is ________.
A) 10.3 mol L-1
B) 1.74 mol L-1
C) 0.465 mol L-1
D) 2.42 × 10-2 mol L-1
E) 9.70 × 10-2 mol L-1
G) A) and E)
Correct Answer

verified
Correct Answer
verified