A) 0.048
B) 11
C) 8.4
D) 3.6
E) 8.2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The data in the table below were obtained for the reaction:
A + B → P  -The order of the reaction in A is .
-The order of the reaction in A is .
A) 1
B) 2
C) 3
D) 4
E) 0
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The mechanism for formation of the product X is:
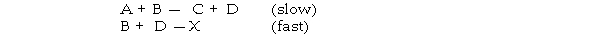 The intermediate reactant in the reaction is .
The intermediate reactant in the reaction is .
A) A
B) B
C) C
D) D
E) X
Correct Answer

verified
Correct Answer
verified
True/False
The rate limiting step in a reaction is the slowest step in the reaction sequence.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A reaction was found to be third order in A. Increasing the concentration of A by a factor of 3 will cause the reaction rate to .
A) remain constant
B) decrease by a factor of the cube root of 3
C) increase by a factor of 9
D) increase by a factor of 27
E) triple
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
The decomposition of N2O5 in solution in carbon tetrachloride proceeds via the reaction 2N2O5 (soln) → 4NO2 (soln) + O2 (soln) The reaction is first order and has a rate constant of 4.82 × 10- 3 s- 1 at 64°C. If the reaction is initiated with 0.058 mol in a 1.00- L vessel, how many moles remain after 151 s?
A) 0.055
B) 2.0 × 103
C) 0.028
D) 0.060
E) 12
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
In the Arrhenius equation, k = Ae- Ea/RT Is the frequency factor.
A) R
B) A
C) Ea
D) e
E) k
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
The reaction below is first order in [H2O2]: 2H2O2 (l) - 2H2O (l) + O2 (g) A solution originally at 0.600 M H2O2 is found to be 0.075 M after 54 min. The half- life for this reaction is min.
A) 28
B) 6.8
C) 18
D) 14
E) 54
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following reaction is second order in [A] and the rate constant is 0.039 M- 1s- 1: A - B The concentration of A was 0.30 M at 23s. The initial concentration of A was _ M.
A) 2.4
B) 1.2 × 10- 2
C) 3.7
D) 0.41
E) 0.27
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The concentration of S2O82- remaining at 1600 s is M.
A) 0.014
B) 0.043
C) 0.029
D) 0.064
E) 0.036
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The rate constant of a first- order process that has a half- life of 225 s is s- 1.
A) 3.08 × 10- 3
B) 0.693
C) 1.25
D) 12.5
E) 4.44 × 10- 3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g) -B(g) . The following data are obtained for [A] as the reaction proceeds: ![A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g) -B(g) . The following data are obtained for [A] as the reaction proceeds: -The average rate of disappearance of A between 10 s and 20 s is mol/s. A) 1.1 × 10<sup>-</sup><sup> </sup><sup>3</sup> B) 4.4 × 10<sup>-</sup><sup> </sup><sup>3</sup> C) 9.90 × 10<sup>- </sup><sup>3</sup> D) 454 E) 2.2 × 10<sup>- </sup><sup>3</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB1819/11ead62c_1f6a_a804_ac95_31c6da222cb4_TB1819_00.jpg) -The average rate of disappearance of A between 10 s and 20 s is mol/s.
-The average rate of disappearance of A between 10 s and 20 s is mol/s.
A) 1.1 × 10- 3
B) 4.4 × 10- 3
C) 9.90 × 10- 3
D) 454
E) 2.2 × 10- 3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The data in the table below were obtained for the reaction:
A + B → P  -The overall order of the reaction is .
-The overall order of the reaction is .
A) 1
B) 2
C) 3
D) 4
E) 0
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the elementary reaction ![For the elementary reaction the molecularity of the reaction is , and the rate law is rate = . A) 2, k[NO<sub>3</sub>][CO]/[NO<sub>2</sub>][CO<sub>2</sub>] B) 2, k[NO<sub>2</sub>][CO<sub>2</sub>] C) 4, k[NO<sub>3</sub>][CO][NO<sub>2</sub>][CO<sub>2</sub>] D) 4, k[NO<sub>2</sub>][CO<sub>2</sub>]/[NO<sub>3</sub>][CO] E) 2, k[NO<sub>3</sub>][CO]](https://d2lvgg3v3hfg70.cloudfront.net/TB1819/11ead62c_1f69_4871_ac95_2bd23521ed09_TB1819_00.jpg) the molecularity of the reaction is , and the rate law is rate = .
the molecularity of the reaction is , and the rate law is rate = .
A) 2, k[NO3][CO]/[NO2][CO2]
B) 2, k[NO2][CO2]
C) 4, k[NO3][CO][NO2][CO2]
D) 4, k[NO2][CO2]/[NO3][CO]
E) 2, k[NO3][CO]
Correct Answer

verified
Correct Answer
verified
True/False
The overall reaction order is the sum of the orders of each reactant in the rate law.
Correct Answer

verified
Correct Answer
verified
Short Answer
If a rate law is second order (reactant) , doubling the reactant _ _ the reaction rate.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The isomerization of methylisonitrile to acetonitrile CH3NC (g) → CH3CN (g) Is first order in CH3NC. The rate constant for the reaction is 9.45 × 10- 5 s- 1 at 478 K. The half- life of the reaction when the initial [CH3NC] is 0.030 M is s.
A) 3.53E × 105
B) 1.36 × 10- 4
C) 7.33 × 103
D) 1.06 × 104
E) 5.29 × 103
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A reaction was found to be second order in carbon monoxide concentration. The rate of the reaction If the [CO] is doubled, with everything else kept the same.
A) doubles
B) remains unchanged
C) is reduced by a factor of 2.
D) increases by a factor of 4
E) triples
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which energy difference in the energy profile below corresponds to the activation energy for the forward reaction?
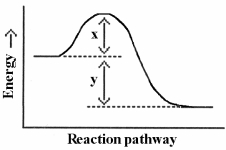
A) x
B) y
C) x - y
D) x + y
E) y - x
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A particular first- order reaction has a rate constant of 1.35 × 102 s- 1 at 25°C. What is the magnitude of k at 95°C if Ea = 55.5 kJ/mol?
A) 4.33 × 1087
B) 1.36 × 102
C) 576
D) 2.85 × 104
E) 9.60 × 103
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 110
Related Exams