A) RCOH
B) ROOC
C) RCOOR
D) RCOOH
E) RCOHO
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When a carboxylic acid dissolves in water, the ion shown below is formed.What is the name of this type of ion? 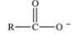
A) enolate ion
B) acyl anion
C) carboxylate anion
D) oxalate ion
E) acetate ion
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following types of compounds can react with a carboxylic acid to form an ester?
A) acyl chloride
B) acetate
C) alcohol
D) ether
E) aldehyde
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which term is used to describe the hydrolysis of an ester by an aqueous base?
A) esterification
B) isomerization
C) enolization
D) tautomerization
E) saponification
Correct Answer

verified
Correct Answer
verified
True/False
Carboxylic acids are too weak to neutralize strong bases such as NaOH.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What two compounds will react to produce methyl hexanoate in an acid catalyzed esterification reaction?
A) methane and hexane
B) methanoic acid and hexanoic acid
C) hexanol and methanol
D) hexanoic acid and methanol
E) hexanol and methanoic acid
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the name of the missing product in the reaction shown below? acetic acid + sodium hydroxide → ? + water
A) sodium acetate
B) sodium chloride
C) acetic hydroxide
D) acetal
E) sodium acetic acid
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What type of organic compound is produced in the reaction below? 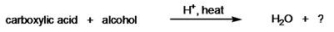
A) ester
B) enol
C) carboxylate salt
D) anhydride
E) acetal
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A cyclic ester (lactone) is formed when a carboxylic acid reacts with an alcohol functional group that is present in the same molecule.Which structure represents the lactone formed in the reaction below? 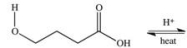
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds would have the greatest solubility in water?
A) ![]()
B) ![]()
C) ![]()
D) HOCH2CH2CH2CH2CH3
E) All would have the same solubility.
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
What carboxylic acid, first synthesized by Bayer and Company, and a derivative of an extract of willow bark, launched the pharmaceutical industry?
A) tartaric acid
B) oxalic acid
C) acetylsalicylic acid
D) benzoic acid
E) salicylic acid
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name of the following compound? 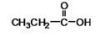
A) ethanoic acid
B) lactic acid
C) pyruvic acid
D) butyric acid
E) propanoic acid
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What role does acetyl coenzyme A (acetyl CoA) play in the body?
A) It serves as the energy source for energy-requiring reactions.
B) It serves as a carrier of two-carbon acetyl groups.
C) It serves as an identification marker on the surface of cells.
D) It functions as a catalyst for reduction reactions.
E) It is a structural component of muscle and bone.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds would react similarly because they contain the same functional group? 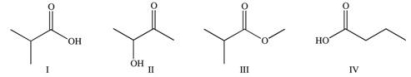
A) I and II
B) I and IV
C) II and IV
D) I, II, and III
E) I, II, and IV
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
What is the IUPAC name of the following compound? 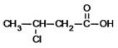
A) 3-chlorobutanoic acid
B) 2-chlorobutanoic acid
C) 2-chloropropanoic acid
D) 3-chloropropanoic acid
E) 3-chloro-1-propanoic acid
Correct Answer

verified
Correct Answer
verified
True/False
A single carboxylic acid molecule may contain more than one carboxyl functional group.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What carboxylic acid is produced in muscle cells during strenuous exercise?
A) adipic acid
B) citric acid
C) oxalic acid
D) lactic acid
E) None of the choices are correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the common name of the acid formed in the following reaction? 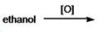
A) formic acid
B) acetic acid
C) oxalic acid
D) methanoic acid
E) propanoic acid
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
What is the IUPAC name of the compound formed by the oxidation of 2-bromopentanal?
A) 2-bromo-1-pentanol
B) 2-bromopentanoate
C) 2-bromopentanoic acid
D) 2-bromo-2-pentanol
E) 2-bromo-2-pentene
Correct Answer

verified
Correct Answer
verified
True/False
The formula of trifluoroacetic acid is CF3COOF.
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 80
Related Exams