A) There is an equal number of protons and neutrons.
B) There is an equal number of protons and electrons.
C) There is an equal number of protons, neutrons, and electrons.
D) The number of protons and neutrons is an even number.
E) The number of protons, neutrons, and electrons is an even number.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which best explains why an Al3+ ion is smaller than an Al atom?
A) In forming the Al3+ ion, the Al atom loses the electrons in its outermost energy level, causing a decrease in the atomic radius.
B) In forming the Al3+ ion, the Al atom gains three protons and the resulting net positive charge keeps the electrons more strongly attracted to the nucleus, reducing the radius.
C) The Al3+ ion contains more electrons than the Al atom, which results in a greater attraction for the nucleus and a smaller atomic radius.
D) In forming the Al3+ ion, the Al atom adds electrons into a higher energy level, causing a decrease in the atomic radius.
E) There are more protons in an Al3+ ion than there are in an Al atom.
Correct Answer

verified
Correct Answer
verified
True/False
In Mendeleev's table, the elements were arranged according to their atomic mass.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many valence electrons are present in a chloride ion, Cl??
A) 5
B) 7
C) 8
D) 17
E) 18
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What can be said about the possibility of the existence of the hydrogen isotope represented by the symbol shown below? 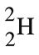
A) This isotope of hydrogen is not possible because it has no electrons.
B) This isotope of hydrogen is not possible because atoms of hydrogen have one proton.
C) This isotope of hydrogen is possible; it simply contains no protons and is an ion.
D) This isotope of hydrogen is possible; it simply contains no neutrons.
E) This isotope of hydrogen is possible; it simply has two neutrons.
Correct Answer

verified
Correct Answer
verified
True/False
Sulfur (S) is one of the representative elements.
Correct Answer

verified
Correct Answer
verified
True/False
Valence electrons are involved when atoms form bonds.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement about the modern periodic table is FALSE?
A) Elements are arranged in order of increasing atomic number.
B) A period is a horizontal row of elements.
C) A group is a vertical column of elements.
D) A stepwise line separates the metals from the nonmetals; metals are to the left of the line, nonmetals are to the right of the line.
E) Elements in the same period share similar chemical and physical properties.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement explains why isotopes have different mass numbers?
A) Isotopes differ in the number of protons each contains.
B) Isotopes differ in the number of electrons each contains.
C) Isotopes differ in the number of neutrons each contains.
D) Isotopes differ in the number of protons and neutrons each contains.
E) Isotopes differ in the number of protons and electrons each contains.
Correct Answer

verified
Correct Answer
verified
True/False
There are eight valence electrons in a chlorine anion.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What property of light is defined by the distance between identical points on adjacent waves?
A) energy
B) speed
C) wavelength
D) spectrum
E) amplitude
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many valence electrons are in Na, O and He, respectively?
A) 1, 6, 8
B) 11, 8, 8
C) 1, 6, 2
D) 11, 8, 2
E) 2, 4, 2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Electron affinity is
A) the energy required to remove an electron from an isolated atom.
B) the force between two electrons in the same orbital.
C) the force between two ions of opposite charge.
D) the energy released when an isolated atom gains an electron.
E) the attraction of an atom for an electron in a chemical bond.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following gives the correct charge of the ion according to the octet rule?
A) F+
B) Ba2-
C) (S2-)
D) P3+
E) C2+
Correct Answer

verified
Correct Answer
verified
True/False
The halogens, Group VII A (17), have the lowest ionization energies of any group in the periodic table.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given that helium has an isotope 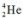 , how many electrons does an atom of this helium isotope contain?
, how many electrons does an atom of this helium isotope contain?
A) 1
B) 2
C) 4
D) 6
E) 0
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which sublevel has the lowest energy?
A) 2s
B) 3p
C) 4p
D) 4s
E) 2p
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Who discovered the existence of the atomic nucleus?
A) Crookes
B) Thomson
C) Geiger
D) Rutherford
E) Bohr
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What kind(s) of particles can be found outside the nucleus of an atom?
A) protons
B) neutrons
C) electrons
D) protons and electrons
E) protons and neutrons
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the general name given to the elements of Group IA (1) ?
A) halogens
B) alkali metals
C) alkaline earth metals
D) noble gases
E) metalloids
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 109
Related Exams