A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following plots indicates that the reaction is zero order?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The energy profiles for four different reactions are shown below. The scales are the same for each. Which of the reactions will have the smallest rate constant?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A proposed mechanism for the decomposition of ozone in the stratosphere is: Step 1: Cl(g) + O3(g) ClO(g) + O2(g) Step 2: ClO(g) + O3(g) Cl(g) + 2 O2(g) What is the molecularity of step 2?
A) unimolecular
B) bimolecular
C) termolecular
D) zero molecular (spontaneous)
E) More information is needed to answer this question.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Large cities often issue ozone advisories. At what time of year would an advisory be likely to occur most often?
A) winter
B) spring
C) summer
D) fall
E) No season should have more advisories than another.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction 2A + 3B 4C + 5D, the rate of the reaction in terms of B would be written as ________
A) - B/ t.
B) + B/ t.
C) -1/3 B/ t.
D) +1/3 B/ t.
E) -3 B/ t.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A scientist conducts an experiment to determine the rate of the following reaction: N2(g) + O2(g) 2NO(g) If the initial concentration of NO was 0.00 M and the concentration of NO was 0.050 M after 0.100 s, what is the average rate of the reaction?
A) 0.500 M/s
B) 1.00 M/s
C) 5.00 M/s
D) 10.0 M/s
E) 0.250 M/s
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The steps in a reaction mechanism are as follows. Which species is acting as a catalyst? Step 1: Ag+(aq) + Ce4+(aq) Ag2+(aq) + Ce3+(aq) Step 2: Tl+(aq) + Ag2+(aq) Tl2+(aq) + Ag+(aq) Step 3: Tl2+(aq) + Ce4+(aq) Tl3+(aq) + Ce3+(aq)
A) Ag+
B) Tl+
C) Ce3+
D) Ag2+
E) Tl3+
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The linear form of ________ is very useful, as it allows us to calculate the activation energy and the frequency factor.
A) the Boltzmann equation
B) the Arrhenius equation
C) Planck's equation
D) the rate law
E) the integrated rate law
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A catalyst that is in a different phase from the reactants and products is a(n) ________ catalyst.
A) heterogeneous
B) homogeneous
C) enzyme-type
D) protein
E) exogenous
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following figure shows Arrhenius plots for four different reactions. Which reaction has the lowest activation energy? 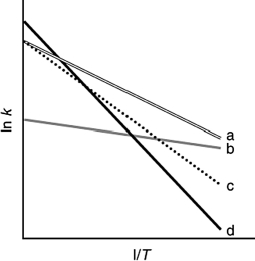
A) a
B) b
C) c
D) d
Correct Answer

verified
Correct Answer
verified
Essay
For the reaction 2A + 3B 4C + 5D, the rate of the reaction in terms of A would be written as ________.
Correct Answer

verified
Correct Answer
verified
Short Answer
Smog created by the interaction of sunlight with nitrogen oxides and volatile organic compounds is termed ________ smog.
Correct Answer

verified
Correct Answer
verified
Short Answer
The device in automobiles that has decreased NO and partially oxidized hydrocarbons from exhaust gases is termed a(n) ________.
Correct Answer

verified
Correct Answer
verified
Essay
For the rate law Rate = k[A][B]3/2, the partial order with respect to A is ________, the partial order with respect to B is ________, and the total order is ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
One reaction that occurs in an automobile catalytic converter is the conversion of nitrogen monoxide to nitrogen and oxygen. How could the rate of this reaction be expressed correctly in terms of the rate at which the concentration of a reactant or product changes? 2NO(g) N2(g) + O2(g)
A) Rate = [NO]/ t
B) Rate = [O2]/ t
C) Rate = 2 [O2]/ t
D) Rate = -2 [NO]/ t
E) Rate = 2 [NO]/ t
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The energy profiles for four different reactions are shown below. The scales are the same for each. Which of the reactions will have the largest rate constant? 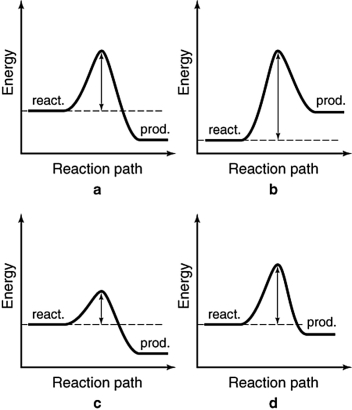
A) a
B) b
C) c
D) d
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the rate law Rate = k[A]3/2[B], the partial order with respect to A is ________, the partial order with respect to B is ________, and the total order is ________.
A)
B)
C)
D)
E) The orders cannot be determined without a chemical reaction.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the following data, determine the order of the reaction with respect to Cl2. 2NO(g) + Cl2(g) 2NOCl(g)
A) first
B) second
C) third
D) fourth
E) fifth
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following plots indicates that the reaction is second order?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Showing 121 - 140 of 164
Related Exams