A) 566.60 kJ
B) 50.38 kJ
C) 25.19 kJ
D) -25.19 kJ
E) -566.60 kJ
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is necessary for a process to be spontaneous?
A) ( Hsys < 0)
B) ( Ssys > 0)
C) ( Ssurr< 0)
D) ( Suniv > 0)
E) ( Gsys = 0)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In order for a process to be spontaneous,
A) ( H must be less than zero.)
B) ( S must be greater than zero.)
C) ( G must be greater than zero.)
D) it should be rapid.
E) ( Ssys + Ssurr must be greater than zero.)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The formation constant for the reaction Ag+(aq) + 2NH3(aq)  Ag(NH3) 2+(aq) is Kf = 1.7 *107 at 25 C. What is G at this temperature?
Ag(NH3) 2+(aq) is Kf = 1.7 *107 at 25 C. What is G at this temperature?
A) -1.5 kJ
B) -3.5 kJ
C) -18 kJ
D) -23 kJ
E) -41 kJ
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Sulfuryl dichloride is formed when sulfur dioxide reacts with chlorine. The data refer to 298 K. 
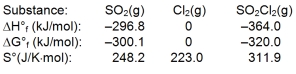 What is the value of G for this reaction at 600 K?
What is the value of G for this reaction at 600 K?
A) -162.8 kJ
B) -40.1 kJ
C) -28.4 kJ
D) 28.4 kJ
E) 162.8 kJ
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For a chemical reaction to be non-spontaneous at any temperature, which of the following conditions must be met?
A) ( S > 0, H > 0)
B) ( S > 0, H < 0)
C) ( S < 0, H < 0)
D) ( S < 0, H > 0)
E) All reactions are spontaneous at some temperature.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the figure below which shows G for a chemical process plotted against absolute temperature. 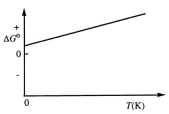 From this plot, it is reasonable to conclude that:
From this plot, it is reasonable to conclude that:
A) ( H > 0, S > 0)
B) ( H > 0, S < 0)
C) ( H < 0, S > 0)
D) ( H < 0, S < 0)
E) None of these choices is correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When a sky diver free-falls through the air, the process is
A) non-spontaneous because he is accelerating due to the force applied by gravity.
B) non-spontaneous because he is losing potential energy.
C) non-spontaneous, if he had planned the jump for two weeks.
D) spontaneous.
E) in equilibrium.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Nitric oxide reacts with chlorine to form NOCl. The data refer to 298 K. 
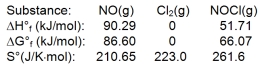 What is the value of G for this reaction at 550 K?
What is the value of G for this reaction at 550 K?
A) -143.76 kJ
B) -78.78 kJ
C) -22.24 kJ
D) -10.56 kJ
E) 66600 kJ
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is true for pure oxygen gas, O2(g) at 25 C?
A) ( H f > 0)
B) ( H f < 0)
C) ( G f > 0)
D) ( G f < 0)
E) (S > 0)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is always true for an exothermic process?
A) qsys > 0, Ssurr < 0
B) qsys < 0, Ssurr > 0
C) qsys < 0, Ssurr < 0
D) qsys > 0, Ssurr > 0
E) w < 0
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which relationship best describes S for the following reaction? CO(g) + H2O(g) CO2(g) + H2(g)
A) ( S = H )
B) ( S = H T)
C) ( S > 0)
D) ( S < 0)
E) ( S 0)
Correct Answer

verified
Correct Answer
verified
True/False
In some spontaneous processes, the entropy of the surroundings decreases.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate S for the combustion of propane. 
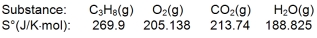
A) -100.9 J/K
B) -72.5 J/K
C) 72.5 J/K
D) 100.9 J/K
E) 877.5 J/K
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is always true for an endothermic process?
A) qsys > 0, Ssurr < 0
B) qsys < 0, Ssurr > 0
C) qsys < 0, Ssurr < 0
D) qsys > 0, Ssurr > 0
E) w < 0
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate S for the reaction 

A) -118.2 J/K
B) -104.8 J/K
C) 104.8 J/K
D) 118.2 J/K
E) 1270.0 J/K
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate S for the reaction 
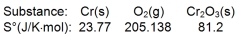
A) -548.1 J/K
B) -147.7 J/K
C) 147.7 J/K
D) 310.1 J/K
E) 548.1 J/K
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which relationship best describes S for the following reaction? 8H2(g) + S8(s) 8H2S(g)
A) ( S = H )
B) ( S = H /T)
C) ( S 0)
D) ( S < 0)
E) ( S > 0)
Correct Answer

verified
Correct Answer
verified
Short Answer
Iron(III) oxide can be reduced by carbon monoxide.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A certain process has Suniv > 0 at 25 C. What does one know about the process?
A) It is exothermic.
B) It is endothermic.
C) It is spontaneous at 25 C.
D) It will move rapidly toward equilibrium.
E) None of these choices is correct.
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 85
Related Exams