A) P
B) Na
C) I
D) B
E) O
Correct Answer

verified
Correct Answer
verified
Essay
Draw a 2p orbital.
Correct Answer

verified
Correct Answer
verified
Essay
Expand the condensed structure shown below to show the covalent bonds and the lone-pair electrons.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many nonbonding pairs of electrons are H2NOH
A) 0
B) 1
C) 2
D) 3
E) 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds have bonds that are predominantly ionic?
A) KCl
B) CF4
C) NH3
D) both A and B
E) both B and C
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
The lone-pair electrons of the methyl anion occupy a(n) ________ orbital.
A) s
B) p
C) sp
D) sp2
E) sp3
Correct Answer

verified
Correct Answer
verified
Essay
Draw the structure of a compound that contains only carbon and hydrogen atoms and that has two sp2 carbons and one sp carbon.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What element is represented by the following electronic configuration?
A) F
B) C
C) N
D) Al
E) O
Correct Answer

verified
Correct Answer
verified
Short Answer
What is the hybridizations of the carbons, going from left to right, in
Correct Answer

verified
sp3, sp2, sp2
Correct Answer
verified
Essay
Why is the shorter and stronger than the bond in ethane (
Correct Answer

verified
The length and strength of a  bond depen...
bond depen...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which of the following compounds does not have a dipole moment of zero?
A) CO2
B) CH4
C) CCl4
D) H2O
E) SO3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which carbons in the following compound are sp hybridized? 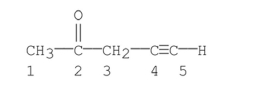
A) carbon 1
B) carbon 2
C) carbons 1, 3
D) carbons 4
E) carbons 4, 5
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following Lewis structures is correct for ?
A)
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Essay
How many nonbonding electron pairs, bonding electron pairs, pi bonds, and sigma bonds are present CO2?
Correct Answer

verified
4 nonbonding electro...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Are all the carbons in this structure in the same plane? 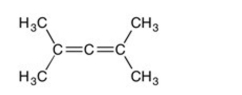
A) Yes
B) No
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds does not have a dipole moment of zero?
A) CH3NH2
B) CO2
C) CH3OCH3
D)
E) CHCl3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the H-C-H bond angle in H2CO
A) 60°
B) 90°
C) 109.5°
D) 120°
E) 180°
Correct Answer

verified
Correct Answer
verified
Essay
Draw the Kekul  structure o CH2Cl2 and show the direction of its dipole moment.
structure o CH2Cl2 and show the direction of its dipole moment.
Correct Answer

verified
11eb4044_1e03_a066_ad6e_f9503501849a_TB1829_00
Correct Answer
verified
Multiple Choice
Each lone pair on the CH3OH occupies a(n) ________ orbital.
A) s
B) p
C) sp
D) sp2
E) sp3
Correct Answer

verified
Correct Answer
verified
Essay
Draw the Lewis structure fo CH3N2+
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 74
Related Exams