A) Potassium sulfate, K 2SO 4
B) Ammonium nitrate, NH 4NO 3
C) Chloromethane, CH 3Cl
D) Calcium chloride, CaCl 2
E) Ethanol, C 2H 6O
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the classification for the following reaction. NH3(aq) + HNO3(aq) → NH4NO3(aq)
A) Precipitation
B) Acid-base
C) Redox
D) Decomposition
E) None of these choices are correct.
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Calculate the oxidation number of the chlorine in perchloric acid, HClO4, a strong oxidizing agent.
A) −1
B) +4
C) +5
D) +7
E) None of these choices are correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the molarity of a 23.55-mL solution which contains 28.24 mg of sodium sulfate (used in dyeing and printing textiles, ℳ = 139.04 g/mol) .
A) 8.625 M
B) 1.199 M
C) 0.8339 M
D) 0.2031 M
E) 0.008625 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which, if any, of the following properties applies to weak acids?
A) They are strong electrolytes.
B) They are excellent conductors of electricity.
C) When dissolved in water, they do not ionize completely.
D) All of these choices are correct.
E) None of these choices are correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the correct set of products for the following reaction. Ba(OH) 2(aq) + HNO3(aq) →
A) BaN 2( s) + H 2O( l)
B) Ba(NO 3) 2( aq) + H 2O( l)
C) Ba( s) + H 2( g) + NO 2( g)
D) Ba 2O( s) + NO 2( g) + H 2O( l)
E) No reaction occurs.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the precipitate that forms when aqueous lead(II) nitrate reacts with aqueous sodium sulfate.
A) NaNO 3
B) Na 2NO 3
C) PbSO 4
D) Pb 2SO 4
E) PbS
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many mL of concentrated nitric acid (HNO3, 16.0 M) should be diluted with water in order to make 2.00 L of 2.00 M solution?
A) 32.0 mL
B) 62.5 mL
C) 125 mL
D) 250 mL
E) 500 mL
Correct Answer

verified
Correct Answer
verified
True/False
Reduction is the loss of electrons.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the oxidation number of iodine in I2.
A) −1
B) 0
C) +1
D) +7
E) None of these choices are correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the classification for the following reaction. BaCl2(aq) + K2SO4(aq) → BaSO4(s) + 2KCl(aq)
A) Precipitation
B) Acid-base
C) Redox
D) Decomposition
E) None of these choices are correct.
Correct Answer

verified
Correct Answer
verified
True/False
Some covalent compounds dissolve in water to produce conducting solutions.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many moles of H+(aq) ions are present in 750 mL of 0.65 M hydrochloric acid?
A) 1.2 mol
B) 0.98 mol
C) 0.87 mol
D) 0.65 mol
E) 0.49 mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following ionic compounds is soluble in water?
A) Na 2S
B) PbI 2
C) AgCl
D) CuS
E) Ca 3(PO 4) 2
Correct Answer

verified
Correct Answer
verified
True/False
In an acid-base (neutralization) reaction the equivalence point is the point where the indicator changes color.
Correct Answer

verified
False
Correct Answer
verified
Multiple Choice
Which of the following is a weak acid?
A) H 2SO 4
B) HNO 3
C) HF
D) HBr
E) HCl
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the products by completing a balanced equation for the following decomposition reaction. CaCl2(l) 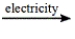 ?
?
A) CaCl 2( l) ![]() Ca( l) + 2Cl -( l)
Ca( l) + 2Cl -( l)
B) CaCl 2( l) ![]() Ca 2+( l) + Cl 2( g)
Ca 2+( l) + Cl 2( g)
C) CaCl 2( l) ![]() Ca 2+( l) + 2Cl -( l)
Ca 2+( l) + 2Cl -( l)
D) CaCl 2( l) ![]() Ca( l) + Cl 2( g)
Ca( l) + Cl 2( g)
E) CaCl 2( l) ![]() CaCl( l) + Cl -( l)
CaCl( l) + Cl -( l)
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
Select the classification for the following reaction. Fe2+(aq) + 2OH-(aq) → Fe(OH) 2(s)
A) Precipitation
B) Acid-base
C) Redox
D) Decomposition
E) None of these choices are correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the product(s) for the following reaction. MgCO3(s) 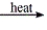
A) MgO 2( s) + CO( g)
B) MgO( s) + CO 2
C) Mg( s) + CO 2( g) + O 2( g)
D) Mg 2+( s) + CO 2( g) + O 2( g)
E) No reaction occurs.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following is a redox reaction?
A) 2Na( g) + Cl 2( g) → 2NaCl( s)
B) Ba 2+( aq) + SO 4 2−( aq) → BaSO 4( s)
C) K 2Cr 2O 7( aq) + 2KOH( aq) → 2K 2CrO 4( aq) + H 2O( l)
D) Na 2CO 3( s) + 2HCl( aq) → 2NaCl( aq) + CO 2( g) + H 2O( l)
E) H 2O( l) → H +( aq) + OH −( aq)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 111
Related Exams