A molecule that contains polar bonds will always have a dipole moment.
B) False
Correct Answer

verified
Correct Answer
verified
Which one of the following Lewis structures is definitely incorrect? 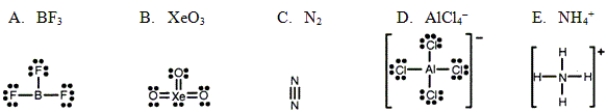
A) A
B) B
C) C
D) D
E) E
G) A) and C)
Correct Answer

verified
Correct Answer
verified