A) 0.9
B) 1.1
C) 2.5
D) Cannot be determined from the information provided.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When an enzyme is saturated with substrates,
A) it will display zero-order kinetics.
B) it will display first-order kinetics.
C) it will display second-order kinetics.
D) it will denature and cease to function.
Correct Answer

verified
Correct Answer
verified
True/False
The substrate will only bind to the enzyme when the shapes fit together rigidly.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What effect is seen on a Lineweaver-Burk graph when a competitive inhibitor is added?
A) The y-intercept is changed, but not change the slope of the line.
B) The slope of the line is changed, but not the y-intercept.
C) Both the y-intercept and the slope of the line are changed.
D) Neither the y-intercept not the slope of the line is changed.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If an inhibitor changes the slope of the Lineweaver-Burk graph,but not the x-intercept,it is this type of inhibition:
A) Competitive.
B) Non-competitive.
C) Mixed Inhibition (uncompetitive inhibition) .
D) You cannot tell from the data given.
E) More than one answer is correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Non-competitive inhibitors have this effect:
A) Modifying the KM value.
B) Changing the value for Vmax.
C) Interfering with substrate binding.
D) This type of inhibitor both changes the Vmax and interferes with substrate binding.
E) All of these are correct.
Correct Answer

verified
Correct Answer
verified
True/False
Thermodynamically favorable reactions all release energy.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A noncompetitive inhibitor
A) binds to the enzyme at a site other than the active site
B) is structurally related to the substrate
C) does not affect the value of Vmax
D) decreases the value of KM
Correct Answer

verified
Correct Answer
verified
True/False
All catalysts work by lowering the activation energy for a reaction.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Exhibit 6A This is a reaction going on in your muscle cells right this very minute:
 The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics:
The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics:
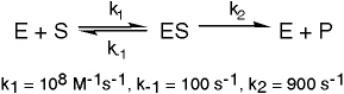 Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A."Restrainin" is an inhibitor of triose phosphate isomerase.When it is added to cells at a concentration of 0.4 nM,the enzyme's apparent KM for the substrate is altered to 100 µM,but the Vmax is unchanged.
Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A."Restrainin" is an inhibitor of triose phosphate isomerase.When it is added to cells at a concentration of 0.4 nM,the enzyme's apparent KM for the substrate is altered to 100 µM,but the Vmax is unchanged.
A) This is a competitive inhibitor.
B) This is an uncompetitive inhibitor.
C) This is a noncompetitive inhibitor.
D) This is an irreversible inhibitor.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The rate of a reaction depends on
A) the free energy change
B) the activation energy
C) the enthalpy change
D) the entropy change
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Competitive inhibitors have this effect:
A) Modifying the KM value.
B) Changing the value for Vmax.
C) Interfering with substrate binding.
D) This type of inhibitor both changes the KM and interferes with substrate binding.
E) All of these are correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to the steady-state assumption
A) the product concentration does not change significantly
B) the substrate concentration is large and does not change significantly
C) the concentration of enzyme-substrate complex remains constant with time
D) the free enzyme concentration is always in great excess to the concentration of enzyme-substrate complex
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The KM of hexokinase for glucose = 0.15 mM and for fructose,KM = 1.5 mM.Which is the preferred substrate?
A) Glucose.
B) Fructose.
C) Neither substrate is preferred over the other.
D) You cannot tell from the data given.
E) None of these answers is correct.
Correct Answer

verified
Correct Answer
verified
True/False
Most enzyme reactions display first order kinetics for the individual substrates when the substrate concentration is low.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The drug acetazolamide:
A) Is used to help fight altitude sickness
B) Was found to ruin the taste of carbonated beverages
C) Does not affect the taste of non-carbonated liquors
D) Causes its effect on taste by inhibiting carbonic anhydrase 4
E) All of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The steady state of an enzyme reaction is the following:
A) The rate observed just after mixing the enzyme and substrate.
B) The rate observed and Vmax.
C) The rate of product formation.
D) The state which exists when E-S complex is forming as fast as it is breaking down.
E) The state which exists when substrate concentration equals KM.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Exhibit 6A This is a reaction going on in your muscle cells right this very minute:
 The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics:
The enzyme triose phosphate isomerase catalyzes this reaction in the forward direction as part of the glycolytic pathway.It follows simple Michaelis-Menten kinetics:
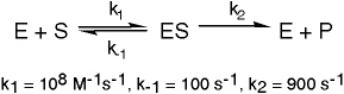 Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A."Hindrate" is an inhibitor of triose phosphate isomerase.When it is added to cells at a concentration of 0.1 nM,the enzyme's KM for the substrate is unchanged,but the apparent Vmax is altered to 50 nM/sec.
In the following graph,which line best represents the Lineweaver-Burk plot obtained in the presence of hindrate?
Typical cellular concentrations: triose phosphate isomerase = 0.1 nM
Dihydroxyacetone phosphate = 5 µM glyceraldehyde-3-phosphate = 2 µM
Refer to Exhibit 6A."Hindrate" is an inhibitor of triose phosphate isomerase.When it is added to cells at a concentration of 0.1 nM,the enzyme's KM for the substrate is unchanged,but the apparent Vmax is altered to 50 nM/sec.
In the following graph,which line best represents the Lineweaver-Burk plot obtained in the presence of hindrate?
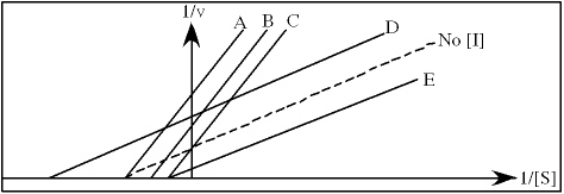
A) A
B) B
C) C
D) D
E) E
Correct Answer

verified
Correct Answer
verified
True/False
The E-S complex often shows as a slight depression in the energy profile for the reaction.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the y-intercept of a Lineweaver-Burk plot = 1.91 (sec/millimole) and the slope = 75.3 L/sec,KM equals:
A) 0.0254 millimolar (mM) .
B) 0.523 millimolar (mM) .
C) 5.23 millimolar (mM) .
D) 39.4 millimolar (mM) .
E) 75.3 millimolar (mM) .
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 78
Related Exams