A) eg = tetrahedral,mg = tetrahedral
B) eg = linear,mg = trigonal planar
C) eg = trigonal planar,mg = bent
D) eg = linear,mg = linear
E) eg = trigonal planar,mg = trigonal planar
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The orbital hybridization on the carbon atoms in C2H2 is ________.
A) sp
B) sp2
C) sp3
D) sp3d2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the electron geometry,molecular geometry and polarity of TeCl6.
A) eg = octahedral,mg = octahedral,nonpolar
B) eg = trigonal bipyramidal,mg = trigonal bipyramidal,nonpolar
C) eg = octahedral,mg = square planar,polar
D) eg = trigonal bipyramidal,mg = see-saw,polar
E) eg = tetrahedral,mg = trigonal pyramidal,polar
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the electron geometry (eg) and molecular geometry (mg) of CO32⁻.
A) eg = tetrahedral,mg = tetrahedral
B) eg = tetrahedral,mg = trigonal pyramidal
C) eg = trigonal planar,mg = bent
D) eg = trigonal planar,mg = trigonal planar
E) eg = tetrahedral,mg = trigonal planar
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using the VSEPR model,the molecular geometry of the central atom in NCl3 is ________.
A) linear
B) trigonal planar
C) tetrahedral
D) bent
E) trigonal pyramidal
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using the VSEPR model,the molecular geometry of the central atom in BF3 is ________.
A) linear
B) trigonal planar
C) tetrahedral
D) bent
E) trigonal pyramidal
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the electron geometry (eg) and molecular geometry (mg) of BrF3.
A) eg = trigonal planar,mg = trigonal planar
B) eg = trigonal bipyramidal,mg = T-shape
C) eg = trigonal planar,mg = bent
D) eg = trigonal bipyramidal,mg = see-saw
E) eg = tetrahedral,mg = trigonal pyramidal
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular geometry of SBr4?
A) seesaw
B) square planar
C) square pyramidal
D) tetrahedral
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using the VSEPR model,the molecular geometry of the central atom in CF4 is ________.
A) linear
B) trigonal planar
C) tetrahedral
D) bent
E) trigonal pyramidal
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the molecule below.Determine the hybridization at each of the three atoms (C,C,N) from left to right 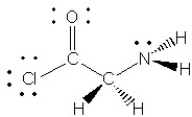
A) 1 = sp2,2 = sp3,3 =s p2
B) 1 = sp2,2 = sp3,3 = sp3
C) 1 = sp3,2 = sp3,3 = sp3
D) 1 = sp3,2 = sp3,3 = sp2
E) 1 = sp,2 = sp2,3 = sp2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to the MO theory,which of the following molecules should not exist?
A) He2+
B) He2
C) H2+
D) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the electron geometry (eg) and molecular geometry (mg) of SiF4.
A) eg = tetrahedral,mg = trigonal pyramidal
B) eg = octahedral,mg = square planar
C) eg = trigonal bipyramidal,mg = trigonal pyramidal
D) eg = tetrahedral,mg = bent
E) eg = tetrahedral,mg = tetrahedral
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many of the following molecules have sp3d hybridization on the central atom? SiCl4 BrF5 AsF5 BrF3
A) 2
B) 0
C) 4
D) 1
E) 3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the molecule below.Determine the hybridization at each of the 2 labeled carbons. 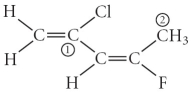
A) C1 = sp3,C2 = sp3d
B) C1 = sp,C2 = sp2
C) C1 = sp2,C2 = sp3d
D) C1 = sp3d,C2 = sp3d2
E) C1 = sp2,C2 = sp3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular geometry of SF5-?
A) octahedral
B) seesaw
C) square pyramidal
D) trigonal bipyramidal
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Place the following in order of increasing dipole moment. I.BCl3 II.BIF2 III.BClF2
A) I < II = III
B) II < III < I
C) I < II < III
D) II < I < III
E) I < III < II
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Draw the Lewis structure for BrF4-.What is the hybridization on the Br atom?
A) sp3d2
B) sp3d
C) sp3
D) sp2
E) sp
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many of the following molecules have sp2 hybridization on the central atom? HCN SO2 OCl2 XeCl2
A) 4
B) 3
C) 2
D) 1
E) 0
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the bond order in He2 molecule?
A) 0
B) 1
C) 1/2
D) 2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the electron geometry (eg) and molecular geometry (mg) of XeF4.
A) eg = tetrahedral,mg = tetrahedral
B) eg = linear,mg = linear
C) eg = tetrahedral,mg = bent
D) eg = trigonal bipyramidal,mg = tetrahedral
E) eg = octahedral,mg = square planar
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 109
Related Exams