A) 0.186 L
B) 0.251 L
C) 0.383 L
D) 0.410 L
E) 0.517 L
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of a gas occupies 1.40 L at 25°C and 760 mmHg.What volume will it occupy at the same temperature and 380 mmHg?
A) 2.8 L
B) 2.10 L
C) 1.40 L
D) 1.05 L
E) 0.700 L
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The pressure of hydrogen sulfide gas in a container is 3.565 ×104 Pa.What is this pressure in torr? (1 atm = 101,325 Pa = 760 torr) ?
A) 46.91 torr
B) 267.4 torr
C) 351.8 torr
D) 3612 torr
E) 2.709 ×104 torr
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of a gas has an initial pressure of 0.987 atm and a volume of 12.8 L.What is the final pressure if the volume is increased to 25.6 L?
A) 2.03 atm
B) 1.97 atm
C) 0.494 atm
D) 0.003 atm
E) 323.4 atm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If 25.5 L of oxygen are cooled from 150°C to 50°C at constant pressure, what is the new volume of oxygen?
A) 0.0514 L
B) 19.5 L
C) 33.4 L
D) 0.03 L
E) 3.5 L
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the formula which describes the relationship between the pressure, volume, temperature, and moles? 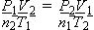
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of carbon dioxide gas at 125°C and 248 torr occupies a volume of 275 L.What will the gas pressure be if the volume is increased to 321 L at 125°C?
A) 212 torr
B) 289 torr
C) 356 torr
D) 441 torr
E) 359 torr
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the molar mass of Freon-11 gas if a sample weighing 0.597 g occupies 100 cm3 at 95°C and 1000 mmHg (R = 0.08206 L • atm/K • mol, 1 atm = 760 mmHg) .
A) 0.19 g/mol
B) 35.3 g/mol
C) 70.9 g/mol
D) 137 g/mol
E) 384 g/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the initial pressure of a gas having an initial temperature of 905 K, an initial volume of 14.3 L, a final pressure of 0.83 atm, a final temperature of 154 K and a final volume of 2.7 L?
A) 26 atm
B) 0.27 atm
C) 0.75 atm
D) 1.3 atm
E) 0.92 atm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The pressure of sulfur dioxide in a container is 159 kPa.What is this pressure in atmospheres? (1 atm = 101,325 Pa = 760 torr) ?
A) 0.209 atm
B) 0.637 atm
C) 1.57 atm
D) 21.2 atm
E) 1.59×104 atm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the partial pressure of N2 in a gas mixture composed of 1.0 mol of N2 and 1.5 mol of Ar.The mixture is at 0.0ºC in a 10.0-liter container.
A) 3.36 atm
B) 0.00 atm
C) 5.60 atm
D) 6.12 atm
E) 2.24 atm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Gases are sold in large cylinders for laboratory use.What pressure is exerted by 2.500 kg of oxygen gas (O2) stored at 22°C in a 40.0-L cylinder? (R = 0.08206 L • atm/K • mol)
A) 3.55 atm
B) 1.51 ×103 atm
C) 47.3 atm
D) 7.56 × 104 atm
E) 10.2 atm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the mole fraction of CO2 in a gas mixture composed of 10.0 grams of CO2 and 10.0 grams of helium.The mixture is at 75ºC in a 12.0-liter container.
A) 0.92
B) 2.7
C) 0.23
D) 0.080
E) 0.77
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molar mass of an unknown gas if a sample weighing 0.389 g is collected in a flask with a volume of 102 cm3 at 97°C and at a pressure of 728 mmHg? (R = 0.08206 L • atm/K • mol, 1 atm = 760 mmHg) .
A) 187 g/mol
B) 121 g/mol
C) 112 g/mol
D) 31.6 g/mol
E) 8.28 × 10−3 g/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A gas cylinder containing 1.50 mol compressed methane has a volume of 3.30 L.What pressure does the methane exert on the walls of the cylinder if its temperature is 25°C? (R = 0.08206 L • atm/K • mol)
A) 9.00 × 10−2 atm
B) 0.933 atm
C) 1.11 atm
D) 1.70 atm
E) 11.1 atm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A gas evolved during the fermentation of sugar was collected at 22.5°C and 702 mmHg.After purification its volume was found to be 25.0 L.How many moles of gas were collected? (R = 0.08206 L • atm/K • mol, 1 atm = 760 mmHg)
A) 0.952 mol
B) 1.05 mol
C) 12.5 mol
D) 22.4 mol
E) 724 mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many molecules of N2 gas are present in a 2.5-L flask at 50°C and 650 mmHg? (R = 0.08206 L • atm/K • mol, 1 atm = 760 mmHg, 1 mole = 6.022 × 1023 molecules)
A) 2.1 × 10−23 molecules
B) 4.9 × 1022 molecules
C) 3.1 × 1023 molecules
D) 3.6 × 1025 molecules
E) 0.081 molecules
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the partial pressure of helium in a gas mixture composed of 10.0 grams of CO2 and 10.0 grams of helium.The mixture is at 75ºC in a 12.0-liter container.
A) 5.95 atm
B) 1.28 atm
C) 0.541 atm
D) 0.117 atm
E) 7.23 atm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of nitrogen gas has a volume of 32.4 L at 20°C.The gas is heated to 220ºC at constant pressure.What is the final volume of the nitrogen gas?
A) 2.94 L
B) 19.3 L
C) 32.4 L
D) 54.5 L
E) 356 L
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of propane, a component of LP gas, has a volume of 35.3 L at 315 K and 922 torr.What is its volume at STP? (R = 0.08206 L • atm/K • mol, 1 atm = 760 torr)
A) 25.2 L
B) 30.6 L
C) 33.6 L
D) 37.1 L
E) 49.2 L
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 60
Related Exams