A) 444.5 pm
B) 352.8 pm
C) 280.0 pm
D) 368.2 pm
E) 417.9 pm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Acetic acid has a heat of fusion of 10.8 kJ/mol and a heat of vaporization of 24.3 kJ/mol. What is the expected value for the heat of sublimation of acetic acid?
A) 35.1 kJ/mol
B) -13.5 kJ/mol
C) +13.5 kJ/mol
D) -35.1 kJ/mol
E) Not enough information is given to answer the question.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following would be expected to have the lowest vapor pressure at room temperature?
A) ethanol, bp = 78°C
B) methanol, bp = 65°C
C) water, bp = 100°C
D) acetone, bp = 56°C
Correct Answer

verified
Correct Answer
verified
Short Answer
What phase exists at the point labeled c? 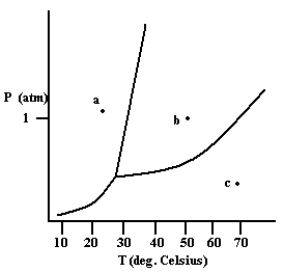
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the amount of heat that must be absorbed by 10.0 g of ice at -20°C to convert it to liquid water at 60.0°C. Given: specific heat (ice) = 2.1 J/g·°C; specific heat (water) = 4.18 J/g·°C; Hfus = 6.0 kJ/mol.
A) 420 J
B) 2,900 J
C) 6,300 J
D) 63 kJ
E) 7.5 J
Correct Answer

verified
Correct Answer
verified
Short Answer
Given the following compound and its boiling point, identify whether it is polar or nonpolar: HCl, -84.9°C.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following substances should exhibit hydrogen bonding in the liquid state?
A) SiH4
B) H2
C) H2S
D) CH4
E) CH3NH2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Atoms share electrons.
A) 1 and 4
B) 1 and 3
C) 2 and 3
D) 2 and 4
E) 3 and 4
Correct Answer

verified
Correct Answer
verified
Short Answer
Indicate all the types of intermolecular forces of attraction in CHCl3(l).
Correct Answer

verified
dipole-dip...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which one of the following is an example of a covalent network solid?
A) SiO2
B) K
C) I2
D) CaCl2
E) None of these.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following substances crystallizes as a covalent crystal?
A) CaO
B) SiO2
C) CO2
D) Pb
E) KMnO4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The heat capacity of liquid water is 4.18 J/g·°C and the heat of vaporization is 40.7 kJ/mol. How many kilojoules of heat must be provided to convert 1.00 g of liquid water at 67°C into 1.00 g of steam at 100°C?
A) 22.7 kJ
B) 40.8 kJ
C) 2.2 kJ
D) 2,400 J
E) 40.8 J
Correct Answer

verified
Correct Answer
verified
Short Answer
Of the pair of compounds given, which would have the stronger intermolecular forces of attraction? CH4 or CH3OH
Correct Answer

verified
Correct Answer
verified
Essay
The meniscus for water is curved upward at the edges (i.e., it is "concave up"). Explain this phenomenon in terms of cohesion and adhesion.
Correct Answer

verified
Adhesion of water to...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which of the following properties indicates the presence of strong intermolecular forces in a liquid?
A) a low heat of vaporization
B) a low critical temperature
C) a low vapor pressure
D) a low boiling point
E) None of the above.
Correct Answer

verified
Correct Answer
verified
Short Answer
Magnesium oxide, MgO, melts at 2,800°C and is very hard. The liquid conducts electricity very well. What kind of crystal is this?
Correct Answer

verified
Correct Answer
verified
Short Answer
Given the following compound and its boiling point, identify whether it is polar or nonpolar: Ar, -185.7°C.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Vanadium crystallizes in a body-centered cubic lattice, and the length of the edge of a unit cell is 305 pm. What is the density of V?
A) 5.96 g/cm3
B) 2.98 g/cm3
C) 2.98 * 10-6 g/cm3
D) 5.96 * 10-30 g/cm3
E) 11.9 g/cm3
Correct Answer

verified
Correct Answer
verified
Showing 121 - 138 of 138
Related Exams