A) figure (2)
B) figure (3)
C) figure (4)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The layers of graphite are held together by
A) covalent bonds.
B) dipole-dipole forces.
C) London dispersion forces.
D) All of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following should have the largest dipole moment?
A) F2(g)
B) BCl3(g)
C) KBr(g)
D) CH3I(g)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A certain mineral crystallizes in the cubic unit cell shown below. 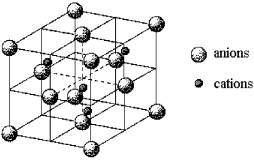 -How many cations and how many anions are in the unit cell?
-How many cations and how many anions are in the unit cell?
A) 4 cations and 4 anions
B) 4 cations and 8 anions
C) 4 cations and 14 anions
D) 8 cations and 4 anions
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the physical phase of the substance at T = 100 K and P = 0.1 atm?
A) gas
B) liquid
C) solid
D) supercritical fluid
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which drawing best represents hydrogen bonding? 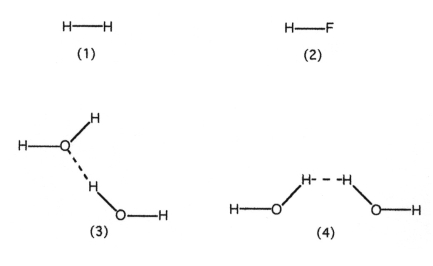
A) drawing (1)
B) drawing (2)
C) drawing (3)
D) drawing (4)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A certain mineral,MxM'yAz,crystallizes in the cubic unit cell shown below.M and M' represent cations and A represents the anions. 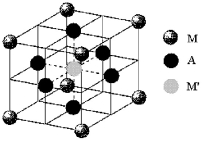 -If cation M has a 2+ charge and anion A has a 2- charge,what is the oxidation state of cation M'?
-If cation M has a 2+ charge and anion A has a 2- charge,what is the oxidation state of cation M'?
A) +1
B) +2
C) +3
D) +4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Manganese crystallizes in a body-centered cubic structure.What is the coordination number of each atom?
A) 4
B) 6
C) 8
D) 12
Correct Answer

verified
Correct Answer
verified
Short Answer
Ni has a face-centered unit cell.The number of Ni atoms in the unit cell is ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
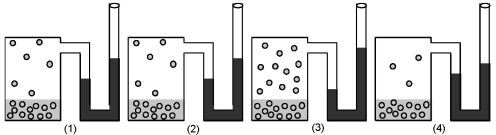 -If figure (1) represents the vapor pressure of water at 25°C,which figure represents the vapor pressure of water at 45°C?
-If figure (1) represents the vapor pressure of water at 25°C,which figure represents the vapor pressure of water at 45°C?
A) figure (2)
B) figure (3)
C) figure (4)
Correct Answer

verified
Correct Answer
verified
Short Answer
Helium can be liquefied when He atoms are attracted to one another by intermolecular ________ forces.
Correct Answer

verified
dispersion...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
How many atoms are in one face-centered cubic unit cell of a metal?
A) 1
B) 2
C) 3
D) 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
As a liquid evaporates at a temperature below its boiling point,the temperature of the liquid
A) decreases.
B) decreases at low temperatures,but increases at high temperatures.
C) increases.
D) remains unchanged.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
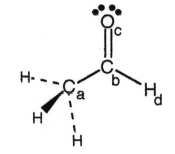 -In the drawing of acetaldehyde,CH3CHO,the largest partial positive charge (δ+) occurs on
-In the drawing of acetaldehyde,CH3CHO,the largest partial positive charge (δ+) occurs on
A) atom (a) .
B) atom (b) .
C) atom (c) .
D) atom (d) .
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many H- ions are around each Na+ ion in NaH,which has a cubic unit cell with H- ions on each corner and each face?
A) 1
B) 4
C) 6
D) 8
Correct Answer

verified
Correct Answer
verified
Multiple Choice
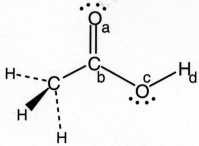 -In the drawing of acetic acid,CH3CO2H,a partial negative charge (δ-) occurs on
-In the drawing of acetic acid,CH3CO2H,a partial negative charge (δ-) occurs on
A) only atom (a) .
B) only atom (b) .
C) atoms (a) and (c) .
D) atoms (b) and (d) .
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The phase diagram of a substance is shown below. 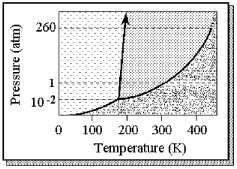 -The approximate normal boiling point of this substance is
-The approximate normal boiling point of this substance is
A) 180 K.
B) 190 K.
C) 300 K.
D) 430 K.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the packing in the figure shown below. 
A) body-centered cubic
B) cubic closest packed (face-centered cubic)
C) hexagonal closest packed
D) simple cubic
Correct Answer

verified
Correct Answer
verified
Multiple Choice
CFC-11 (trichlorofluoromethane,CCl3F) has been used for many years as the working fluid in refrigerators.Given its heat of vaporization is 26.88 kJ/mol and its entropy of vaporization is 90.51 J/(mol ∙ K) ,what is the boiling point of CFC-11?
A) -272.9°C
B) 0) 297°C
C) 2) 44°C
D) 23.8°C
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When a narrow diameter glass tube is inserted into a body of water,water rises in the tube and its surface inside is concave upwards.Which statement,concerning the strength of the intermolecular forces between glass and water molecules compared to those between water molecules,is accurate?
A) The forces of attraction between the glass and water are weaker than those in water.
B) The forces of attraction between the glass and water are stronger than those in water.
C) The forces of attraction between the glass and water are the same as those in water.
D) Intermolecular forces are irrelevant to this situation.
Correct Answer

verified
Correct Answer
verified
Showing 161 - 180 of 186
Related Exams