In the following picture, each arrow represents a molecule or atom.Based on the arrangement in the solid state as shown, which of the following best represents the unit cell? 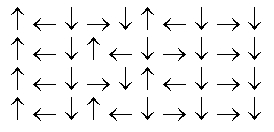
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Which of the following substances is expected to have the highest molar heat of vaporization ( Hvap) ?
A) Ar
B) C6H6
C) He
D) NH3
E) H2O
G) B) and D)
Correct Answer

verified
Correct Answer
verified