Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the pOH for a solution with [H3O+] = 2.5 x 10-5 M
A) 4.60
B) 9.40
C) 4.0 x 10-10
D) 2.50
E) 11.50
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The pH of a 0.14 M solution of an unknown monoprotic acid is 5.85.Calculate the Ka of the acid.
A) 1.4 x 10-6
B) 1.4 x 10-11
C) 1.0 x 10-5
D) 7.1 x 10-9
E) 2.0 x 10-7
Correct Answer

verified
Correct Answer
verified
Short Answer
A 8.0 M solution of formic acid (HCOOH)is 0.47% ionized.What is the Ka of formic acid?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Diet cola drinks have a pH of about 3.0, while milk has a pH of about 7.0.How many times greater is the H3O+ concentration in diet cola than in milk?
A) 2.3 times higher in diet cola than in milk
B) 400 times higher in diet cola than in milk
C) 0.43 times higher in diet cola than in milk
D) 1,000 times higher in diet cola than in milk
E) 10,000 times higher in diet cola than in milk
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not a conjugate acid-base pair?
A) H2O and OH-
B) H2O and H3O+
C) H3O+ and OH-
D) HO2- and H2O2
E) O22- and HO2-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following solutions is basic?
A) [OH-] = 1.0 x 10-14 M
B) [OH-] = 1.0 x 10-7 M
C) [H3O+] = 1.0 x 10-14 M
D) [H3O+] > 1.0 x 10-7 M
E) [OH-] < 1.0 x 10-7 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the pH of a 0.30 M solution of HOCl (Ka = 3.5 x 10-8)
A) 7.45
B) 6.54
C) 7.98
D) 5.22
E) 3.99
Correct Answer

verified
Correct Answer
verified
Short Answer
Write the formula for the conjugate base of H3PO4
Correct Answer

verified
Correct Answer
verified
Short Answer
Will a 0.1 M solution of NH4CN(aq)be acidic, basic, or neutral?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the direction in which the equilibrium will lie for the reaction C6H5COO- + HF 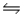 C6H5COOH + F- .
Ka(C6H5COOH) = 6.5 × 10-5; Ka(HF) = 7.1 × 10-4
C6H5COOH + F- .
Ka(C6H5COOH) = 6.5 × 10-5; Ka(HF) = 7.1 × 10-4
A) to the right
B) to the left
C) in the middle
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The OH- concentration in a 1.0 × 10-3 M Ba(OH) 2 solution is
A) 0.50 × 10-3 M.
B) 1.0 × 10-3 M.
C) 2.0 × 10-3 M.
D) 1.0 × 10-2 M.
E) 0.020 M.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A tablet of a common over-the-counter drug contains 200.mg of caffeine (C8H10N4O2) .What is the pH of the solution resulting from the dissolution of two of these tablets in 225.mL of water? (For caffeine, Kb = 4.1 × 10-4.)
A) 2.76
B) 7.67
C) 10.96
D) 6.33
E) 11.24
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the pH of an acid rain storm is approximately 3.0, how many times greater is the [H+] in the rain than in a cup of coffee having a pH of 5.0?
A) 1000
B) 100
C) 20
D) 1.7
E) 0.60
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The pH of a Ba(OH) 2 solution is 10.00.What is the H+ ion concentration of this solution?
A) 4.0 × 10-11 M
B) 1.6 × 10-10 M
C) 1.3 × 10-5 M
D) 1.0 × 10-10 M
E) 10.M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Arrange the acids HBr, H2Se, and H3As in order of increasing acid strength.
A) HBr < H2Se < H3As
B) HBr < H3As < H2Se
C) H2Se < H3As < HBr
D) H3As < H2Se < HBr
E) H3As < HBr < H2Se
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of these statements about strong acids is true?
A) All strong acids have H atoms bonded to electronegative oxygen atoms.
B) Strong acids are 100% ionized in water.
C) The conjugate base of a strong acid is itself a strong base.
D) Strong acids are very concentrated acids.
E) Strong acids produce solutions with a higher pH than weak acids.
Correct Answer

verified
Correct Answer
verified
Short Answer
Calculate the pOH of a solution containing 0.25 g of HCl in 800.mL of solution.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of these salts will form an acidic solution upon dissolving in water?
A) LiBr
B) NaF
C) NH4Br
D) KOH
E) NaCN
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the pOH for a solution with [H3O+] = 3.1 x 10-9 M
A) 3.10
B) 10.90
C) 8.51
D) 5.49
E) 3.2 x 10-6
Correct Answer

verified
Correct Answer
verified
Showing 121 - 140 of 178
Related Exams