A) K2SO4
B) Ca(C2H3O2) 2
C) MgC2O4
D) ZnCl2
E) Mn(NO3) 2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following are strong acids? HI HNO3 HF HBr
A) HF, HBr
B) HI, HNO3, HF, HBr
C) HI, HF, HBr
D) HNO3, HF, HBr
E) HI, HNO3, HBr
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following is a triprotic acid?
A) nitric acid
B) chloric acid
C) phosphoric acid
D) hydrofluoric acid
E) sulfuric acid
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 31.5 mL aliquot of H2SO4 (aq) of unknown concentration was titrated with 0.0134 M NaOH (aq) .It took 23.9 mL of the base to reach the endpoint of the titration.The concentration (M) of the acid was ________.
A) 0.0102
B) 0.00508
C) 0.0204
D) 0.102
E) 0.227
Correct Answer

verified
Correct Answer
verified
Short Answer
What is the concentration (M)of potassium ions in 153 mL of a 1.25 M K3PO4 solution?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When aqueous solutions of AgNO3 and KI are mixed,silver iodide precipitates.The balanced net ionic equation is ________.
A) Ag+ (aq) + ![]() (aq) → AgI (s)
(aq) → AgI (s)
B) Ag+ (aq) + NO3- (aq) → AgNO3 (s)
C) Ag+ (aq) + NO3- (aq) → AgNO3 (aq)
D) AgNO3 (aq) + KI (aq) → AgI (s) + KNO3 (aq)
E) AgNO3 (aq) + KI (aq) → AgI (aq) + KNO3 (s)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What volume (mL) of 0.102 M NaOH is required to neutralize 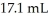 of
of 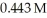 HCl?
HCl?
A) 74.3
B) 3.94
C) 0.773
D) 0.0135
E) 0.000773
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The net ionic equation for the reaction between aqueous solutions of HF and KOH is ________.
A) HF + KOH → H2O + K+ + F-
B) HF + OH- → H2O + F-
C) HF + K+ + OH- → H2O + KF
D) H+ + OH- → H2O
E) H+ + F- + K+ + OH- → H2O + K+ + F-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What volume (L) of 0.250 M HNO3 is required to neutralize a solution prepared by dissolving 17.5 g of NaOH in 350 mL of water?
A) 1.25
B) 0.11
C) 1.75
D) 0.070
E) 1.75 × ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 25.5 mL aliquot of HCl (aq) of unknown concentration was titrated with 0.113 M NaOH (aq) .It took 51.2 mL of the base to reach the endpoint of the titration.The concentration (M) of the acid was ________.
A) 1.02
B) 0.114
C) 0.454
D) 0.113
E) 0.227
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the concentration (M) of sodium ions in a solution made by diluting 50.0 mL of a 0.874 M solution of sodium sulfide to a total volume of 250.0 mL.
A) 0.175
B) 4.37
C) 0.525
D) 0.350
E) 0.874
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the choices below,which would be the best for the lining of a tank intended for use in storage of hydrochloric acid?
A) copper
B) zinc
C) nickel
D) iron
E) tin
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What mass (g) of AgBr is formed when 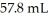 of
of 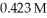 AgNO3 is treated with an excess of aqueous hydrobromic acid?
AgNO3 is treated with an excess of aqueous hydrobromic acid?
A) 4.59
B) 2.30
C) 25.7
D) 0.130
E) 25,700
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The molarity (M) of an aqueous solution containing 129 g of glucose (C6H12O6) in 200 mL of solution is ________.
A) 0.716
B) 0.0036
C) 3.58
D) 0.645
E) 645
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The molarity of a solution prepared by diluting 43.72 mL of 1.005 M aqueous K2Cr2O7 to 500.mL is ________.
A) 0.0879
B) 87.9
C) 0.0218
D) 0.0115
E) 0.870
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The balanced reaction between aqueous nitric acid and aqueous strontium hydroxide is ________.
A) HNO3 (aq) + Sr(OH) 2 (aq) → Sr(NO3) 2 (aq) + H2 (g)
B) HNO3 (aq) + Sr(OH) 2 (aq) → H2O (l) + Sr(NO3) 2 (aq)
C) HNO3 (aq) + SrOH (aq) → H2O (l) + SrNO3 (aq)
D) 2HNO3 (aq) + Sr(OH) 2 (aq) → 2H2O (l) + Sr(NO3) 2 (aq)
E) 2HNO3 (aq) + Sr(OH) 2 (aq) → Sr(NO3) 2(aq) + 2H2 (g)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following solutions will have the greatest concentration of hydroxide ions?
A) 1.10 M rubidium hydroxide
B) 0.368 M calcium hydroxide
C) 0.368 M ammonium hydroxide
D) 0.368 M potassium hydroxide
E) 0.368 M sulfuric acid
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An aqueous ethanol solution (400 mL) was diluted to 4.00 L,giving a concentration of 0.0400 M.The concentration of the original solution was ________ M.
A) 0.400
B) 0.200
C) 2.00
D) 1.60
E) 4.00
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If an unknown sample contains 39.04% sulfuric acid by mass,then a 0.9368 g of that sample would require ________ mL of 0.2389 M NaOH for neutralization.
A) 31.22
B) 39.98
C) 7.80
D) 79.96
E) 15.61
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which reaction does the oxidation number of oxygen increase?
A) Ba(NO3) 2 (aq) + K2SO4 (aq) → BaSO4 (s) + 2KNO3 (aq)
B) HCl (aq) + NaOH (aq) → NaCl (aq) + H2O (l)
C) MgO (s) + H2O (l) → Mg(OH) 2 (s)
D) 2SO2 (g) + O2 (g) → 2SO3 (g)
E) 2H2O (l) → 2H2 (g) + O2 (g)
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 178
Related Exams