A) The atomic spectrum of helium is different from the atomic spectrum of neon.
B) Electromagnetic radiation often behaves like a wave.
C) An atomic spectrum is an element's fingerprint.
D) An atom emits electromagnetic radiation when an electron moves from a lower energy level to a higher energy level.
E) Light that is high in energy has a high frequency.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If an element has 18 protons and 20 neutrons and 18 electrons, which expression correctly identifies the element?
A) argon-38
B) argon-18
C) argon-20
D) calcium-38
E) calcium-20
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following elements is a gas at room temperature?
A) argon (Ar)
B) lead (Pb)
C) cesium (Cs)
D) indium (In)
E) lithium (Li)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If an element has 9 protons and 10 neutrons and 9 electrons, which expression correctly identifies the element?
A) ![]() F
F
B) ![]() F
F
C) ![]() K
K
D) ![]() K
K
E) ![]() K
K
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What does the following element description actually mean? 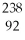 U
U
A) a uranium atom with 92 protons and 146 neutrons
B) a uranium atom with 238 neutrons and 92 protons
C) a uranium atom with 92 neutrons and 238 protons
D) a uranium atom with 92 neutrons and 146 protons
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Since atoms are mostly empty space, why don't objects pass through one another?
A) The electrons on the atoms repel other electrons on other atoms when they get close.
B) The nucleus of one atom repels the nucleus of another atom when it gets close.
C) The nucleus of one atom attracts the nucleus of a neighboring atom to form a barrier.
D) The electrons of one attract the nucleus of a neighboring atom to form a barrier.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements describes an isotope?
A) element with the same number of protons but a different number of neutrons
B) element with the same number of protons but a different number of electrons
C) element with the same number of neutrons but a different number of electrons
D) element with the same number of neutrons but a different number of protons
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not the name of a chemical family?
A) heavy metals
B) transition metals
C) alkali metals
D) alkaline-earth metals
E) noble gases
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following statement describes which subatomic particle best? It does not have an electrical charge.
A) an electron
B) a proton
C) a neutron
D) A and B
E) B and C
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which element has the atomic number 9?
A) F
B) Ne
C) B
D) Na
E) Be
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Does a shell have to contain electrons in order to exist?
A) A shell is a form of energy that requires electrons in order to exist.
B) A shell is just a region of space which may or may not contain electrons.
C) A shell is just a conceptual model, hence, it doesn't really exist with or without the electron.
D) Two of the above are reasonable answers.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An electron de-excites from the fourth quantum level to the third and then directly to the first. Two frequencies of light are emitted. How do their combined energies compare to the energy of the single frequency that would be emitted by de-excitation from the fourth level directly to the first level?The combined energies of the two frequencies emitted by the one electrons is
A) greater than the energy of the single frequency.
B) less than the energy of the single frequency.
C) equal to the energy of the single frequency.
D) not predictable because other factors, such as the temperature of the surroundings must also be considered.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which are older, the atoms in the body of an elderly person or those in the body of a baby?
A) A baby because this is surely a trick question.
B) An elderly person because they have been around much longer.
C) They are of the same age, which is appreciably older than the solar system.
D) It depends upon their diet.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Strontium, Sr (number 38) , is especially dangerous to humans because it tends to accumulate in calcium-dependent bone marrow tissues (calcium, Ca, number 20) . This fact relates to the organization of the periodic table in that strontium and calcium are both
A) metals.
B) in group 2 of the periodic table.
C) made of relatively large atoms.
D) soluble in water.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following elements are in the same group as silicon (Si) ?
A) C
B) P
C) As
D) B
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How does the wave model of electrons orbiting the nucleus account for the fact that the electrons can have only discrete energy values?
A) Electrons are only able to vibrate at particular frequencies.
B) When an electron wave is confined, it is reinforced only at particular frequencies.
C) The energy values of an electron only occur where its wave properties and probability clouds are mutually reinforcing.
D) The wave model accounts for the types of orbitals an electron may occupy, not it's energy levels.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How might the spectrum of an atom appear if its electrons were not restricted to particular energy levels?
A) It would appear nearly the same as it does with the energy level restrictions.
B) There would be no frequencies within the visible portion of the electromagnetic spectrum.
C) A broad spectrum of all colors would be observed.
D) The frequency of the spectral lines would change with temperature.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If a neutral element has the following chemical symbol, how many electrons does it have? 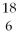 O
O
A) 6
B) 18
C) 12
D) 24
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The isotope lithium-7 has a mass of 7.0160 atomic mass units, and the isotope lithium-6 has a mass of 6.0151 atomic mass units. Given the information that 92.58 percent of all lithium atoms found in nature are lithium-7 and 7.42 percent are lithium-6, calculate the atomic mass of lithium, Li (atomic number 3) .
A) 7.0160 amu
B) 6.942 amu
C) 6.495 amu
D) 13.031 amu
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following diagrams best represents the size of the atomic nucleus relative to the size of the atom? 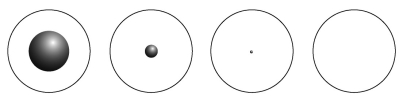 A B C D
A B C D
A) A
B) B
C) C
D) D
Correct Answer

verified
Correct Answer
verified
Showing 101 - 120 of 128
Related Exams