A) requires a catalyst.
B) is endothermic.
C) occurs slowly.
D) is exothermic.
E) cannot occur.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following has a standard enthalpy of formation value of zero at 25°C?
A) I(g)
B) I2(l)
C) I2(s)
D) I(s)
E) I2(g)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine rH for the following reaction,
2 NH3(g) + 5/2 O2(g) 2 NO(g) + 3 H2O(g)
Given the thermochemical equations below.
A) -1178.2 kJ/mol-rxn
B) -452.8 kJ/mol-rxn
C) -394.6 kJ/mol-rxn
D) -211.0 kJ/mol-rxn
E) +1178.2 kJ/mol-rxn
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Heat capacity is defined as
A) the amount of heat required to raise the temperature of 1 gram of substance by 1 K.
B) the amount of heat required to raise the temperature of a substance by 1 K.
C) the amount of heat required to vaporize a solid or liquid.
D) the maximum amount of heat that a substance may absorb without decomposing.
E) 4.18 cal/g.K.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these physical changes would require the release of energy?
A) condensing a gas
B) boiling a liquid
C) melting a solid
D) all of these
E) none of these
Correct Answer

verified
Correct Answer
verified
Short Answer
Dry ice converts directly from a solid to a gas when heated.This process is called ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following processes is/are exothermic? 1) the reaction of butane with oxygen 2) the melting of gold 3) cooling copper from 225 C to 65 C
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 1,2,and 3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using the following thermochemical data:
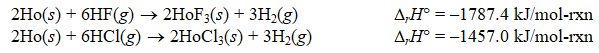 Calculate rH for the following reaction:
HoF3(s) + 3HCl(g) HoCl3(s) + 3HF(g)
Calculate rH for the following reaction:
HoF3(s) + 3HCl(g) HoCl3(s) + 3HF(g)
A) -3244.4 kJ/mol-rxn
B) 330.4 kJ/mol-rxn
C) 165.2 kJ/mol-rxn
D) 660.8 kJ/mol-rxn
E) -1622.2 kJ/mol-rxn
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If 50.0 g of benzene,C6H6,at 25.0 C absorbs 2.71 kJ of energy in the form of heat,what is the final temperature of the benzene? The specific heat capacity of benzene is 1.72 J/g.K.
A) 25.0 C
B) 31.5 C
C) 56.5 C
D) 32.3 C
E) 57.3 C
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following thermodynamic quantities are state functions: heat (q) ,work (w) ,enthalpy change ( H) ,and/or internal energy change ( U) ?
A) q only
B) w only
C) ( H) only
D) ( U) only
E) ( H) and ( U)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the thermochemical equation 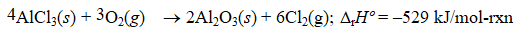 Find rHº for the following reaction.
Find rHº for the following reaction.
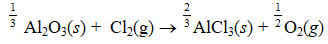
A) (+88.2 kJ/mol-rxn)
B) (+264.5 kJ/mol-rxn)
C) ( +529.0 kJ/mol-rxn)
D) (-176.3 kJ/mol-rxn)
E) (-176.3 kJ/mol-rxn) .
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When 50.0 mL of 1.30 M of HCl(aq) is combined with 50.0 mL of 1.20 M of NaOH(aq) in a coffee-cup calorimeter,the temperature of the solution increases by 8.01°C.What is the change in enthalpy for this balanced reaction? HCl(aq) + NaOH(aq) NaCl(aq) + H2O(l) Assume that the solution density is 1.00 g/mL and the specific heat capacity of the solution is 4.18 J/g. C.
A) -55.8 kJ
B) 55.8 kJ
C) 51.5 kJ
D) -51.5 kJ
E) -26.8 kJ
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When 0.236 mol of a weak base (A-) is reacted with excess HCl,6.91 kJ of energy is released as heat.What is H for this reaction per mole of A- consumed?
A) -34.2 kJ/mol
B) -59.4 kJ/mol
C) -29.3 kJ/mol
D) 34.2 kJ/mol
E) 29.3 kJ/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Specific heat capacity is
A) the quantity of heat needed to change the temperature of 1.00 g of a substance by 1 K.
B) the quantity of heat needed to change the temperature of 1.00 g of a substance by 4.184 K.
C) the capacity of a substance to gain or lose a 1.00 J of energy in the form of heat.
D) the temperature change undergone when 1.00 g of a substance absorbs 4.184 J.
E) the maximum amount of energy in the form of heat that 1.00 g of a substance may absorb without decomposing.
Correct Answer

verified
Correct Answer
verified
Showing 61 - 74 of 74
Related Exams