What is the formal charge on the nitrogen atom in the following compound? 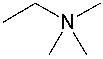
A) -1
B) -2
C) +1
D) +2
E) 0
G) A) and C)
Correct Answer

verified
Correct Answer
verified
How many total lone pairs of electrons are in the following compound? 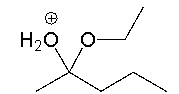
A) one
B) two
C) three
D) four
E) none
G) B) and C)
Correct Answer

verified
Correct Answer
verified