A) NaNH2
B) 1. O3; 2. H2O
C) KMnO4
D) 1. BH3∙THF; 2. H2O2, NaOH
E) H2SO4, H2O
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which sequence of reagents will accomplish the following synthesis? 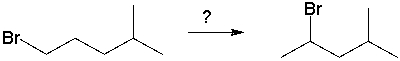
A) 1. KOtBu; 2. HBr
B) 1. NaOEt; 2. HBr, ROOR
C) 1. H2SO4, heat; 2. Br2, h
D) 1. NaOEt; 2. HBr
E) both A and D work
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using retrosynthetic synthesis, determine which compound(s) could lead to the alcohol shown below in a single step. 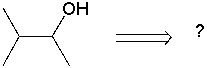
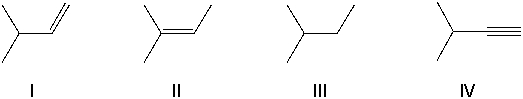
A) I
B) II
C) III
D) IV
E) I or II
Correct Answer

verified
Correct Answer
verified
Multiple Choice
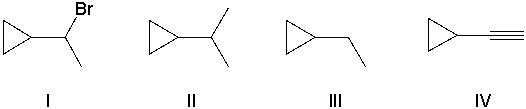
Which of the following alkenes cannot be converted into an alkyne by reacting it with bromine followed by an excess of sodium amide and then with water? 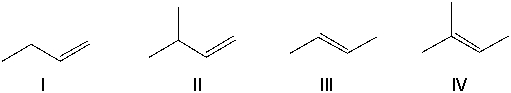
A) I
B) II
C) III
D) IV
E) III and IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the best reagents for the reaction below. 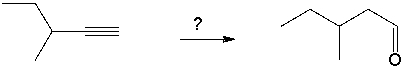
A) 1. OsO4; 2. NaHSO3, H2O
B) 1. Hg(OAc) 2, H2O; 2. NaBH4
C) H2, Pt
D) 1. 9-BBN; 2. H2O2, NaOH
E) 1. O3; 2. DMS
Correct Answer

verified
Correct Answer
verified
Essay
Devise a synthetic route for the following equation. 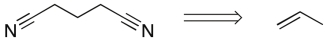
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the best reagents for the reaction below. 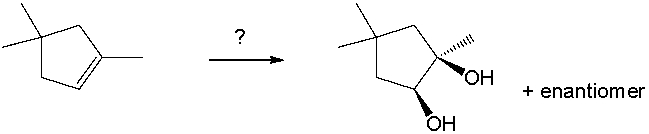
A) 1. OsO4; 2. NaHSO3, H2O
B) 1. Hg(OAc) 2, H2O; 2. NaBH4
C) 1. RCO3H; 2. H3O+
D) H2SO4, H2O
E) 1. O3; 2. DMS
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Provide the major product(s) obtained from the following reaction. 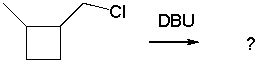
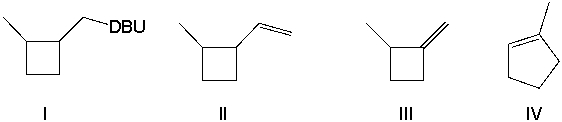
A) I
B) II
C) III
D) IV
E) II and IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Perform a retrosynthetic analysis by working backwards two steps in the synthesis below. Identify possible combinations of A and B that could lead to the alkyl halide (C) . 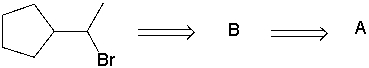
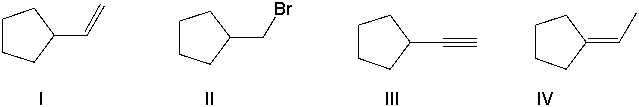
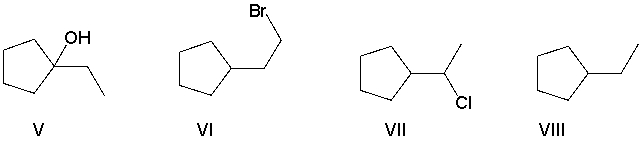
A) B = I and A = VIII
B) B = VI and A = I
C) B = III and A = VII
D) B = IV and A = VII
E) B = V and A = VIII
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using retrosynthetic synthesis, determine which compound(s) could lead to the alkene shown below in a single step. 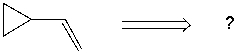
A) I
B) II
C) III
D) IV
E) I or IV
Correct Answer

verified
Correct Answer
verified
Essay
Propose a multi-step synthetic sequence to accomplish the transformation below. 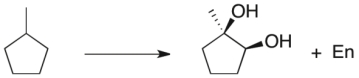
Correct Answer

verified
1. Br2, h...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Compound X has molecular formula C8H10. Reaction of Compound X with excess ozone, followed by reaction with dimethyl sulfide and then washing with water produces only the compounds shown below. Draw a possibility for Compound X that is consistent with these results. 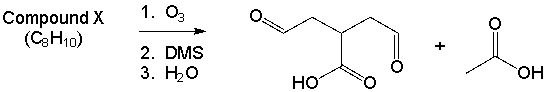
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For multi-step reactions, which is the best method?
A) two steps
B) three steps
C) four steps
D) five steps
E) six steps
Correct Answer

verified
Correct Answer
verified
Essay
Propose a three-step synthetic sequence to accomplish the transformation below. 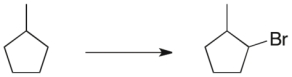
Correct Answer

verified
1. Br...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Identify the statement that is not part of Green Chemistry.
A) Energy efficiency.
B) Renewable feedstocks.
C) Reuse solvents without purification.
D) Prevent waste.
E) Use catalysts, rather that stoichiometric reagents.
Correct Answer

verified
Correct Answer
verified
Essay
Propose a synthesis of cyclopentanone from 1-methylcyclopentane. 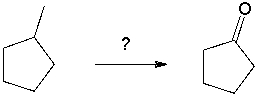
Correct Answer

verified
Correct Answer
verified
Essay
Devise a synthetic route to convert ethylene into PVC (polyvinyl chloride).
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the best reagents for the reaction below. 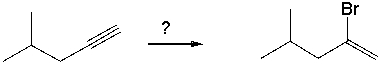
A) 1. OsO4; 2. NaHSO3, H2O
B) HBr, ROOR
C) NaBr
D) excess NaNH2
E) HBr
Correct Answer

verified
Correct Answer
verified
Essay
Propose a three-step synthetic sequence to accomplish the transformation below. 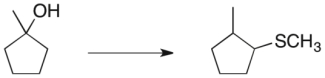
Correct Answer

verified
1. Conc. H...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Propose a synthetic route for the following reaction. 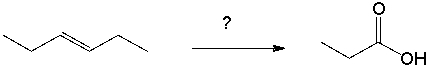
Correct Answer

verified
1. Br2, CCl...View Answer
Show Answer
Correct Answer
verified
View Answer
Showing 81 - 100 of 106
Related Exams