Correct Answer

verified
Correct Answer
verified
Multiple Choice
An atom that has the same number of neutrons as 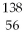 Ba is:
Ba is:
A) ![]()
Cs
B) ![]()
Ba
C) ![]()
La
D) ![]()
Xe
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Cr is a member of which family?
A) noble gases
B) halogens
C) alkaline earth metals
D) alkali metals
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the charge on the ion formed by selenium?
A) 1-
B) 2-
C) 1+
D) 2+
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
A positive charge attracts negative charges and repels other positive charges.
Correct Answer

verified
Correct Answer
verified
True/False
All elements have three or more naturally occurring isotopes.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about ions is INCORRECT?
A) Cations are positive ions and anions are negative ions.
B) Cations are formed when an atom loses electrons.
C) Anions are formed when an atom gains electrons.
D) Cations always have the same number of protons as electrons.
E) All statements are correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the correct chemical symbol for mercury?
A) Hm
B) Hy
C) Me
D) My
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following elements has an atomic number of 4?
A) H
B) C
C) He
D) Be
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about atoms is FALSE?
A) Atoms compose all matter.
B) Atoms are responsible for the sensation of smell.
C) Atoms are the basic building block of nature.
D) An atom is the smallest identifiable unit of an element.
E) All of the above statements are true.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The names of the elements whose symbols are Si,P,Mn,and S are respectively:
A) silicon,potassium,magnesium,and sulfur.
B) silver,phosphorus,magnesium,and sulfur.
C) silicon,phosphorus,manganese,and sulfur.
D) silicon,phosphorus,magnesium,and sulfur.
E) silicon,potassium,magnesium,and sodium.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following elements has only 12 protons?
A) C
B) Zn
C) Mg
D) O
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The number of protons in the nucleus of an atom:
A) is called the atomic number.
B) is given the symbol "Z."
C) identifies the atom as a particular element.
D) is the same for all isotopes of an element.
E) all of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the charge on the cesium ion?
A) 1-
B) 2-
C) 1+
D) 2+
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is NOT a correct name,symbol combination?
A) beryllium,Be
B) magnesium,Mg
C) iron,I
D) manganese,Mn
E) silicon,Si
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about the nature of electrical charge is FALSE?
A) Electrical charge is a fundamental property of protons and electrons.
B) Positive and negative electrical charges attract each other.
C) Positive-positive or negative-negative charges repel each other.
D) Positive and negative charges cancel each other so that a proton and electron,when paired,are charge neutral.
E) All of the above statements are true.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Group 7A elements are also called:
A) noble gases.
B) halogens.
C) alkaline earth metals.
D) alkali metals.
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
Ernest Rutherford proved the existence of electrons.
Correct Answer

verified
Correct Answer
verified
True/False
An atom is the smallest identifiable unit of a compound.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given that the molecular mass of bromine is 79.90 grams,which of the following isotopes would you expect to have the greatest natural abundance?
A) Br-79
B) Br-80
C) Br-81
D) Br-82
E) none of the above
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 112
Related Exams