Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many resonance structures are possible for NO3-?
A) 1
B) 2
C) 3
D) 4
E) 5
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to VSEPR theory, a molecule with the general formula AX2E3 will have a _____ molecular shape.
A) bent
B) linear
C) trigonal planar
D) T-shaped
E) trigonal pyramidal
Correct Answer

verified
Correct Answer
verified
Essay
Explain what is meant by "dipole moment", and give an example of a molecule which has polar bonds but which does not itself have a dipole moment.
Correct Answer

verified
A dipole moment arises in a molecule whe...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Phosphoryl iodide is used in the preparation of organophosphorus derivatives and phosphate esters. Select the Lewis structure for POI3 which minimizes formal charges.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular shape of HOF as predicted by the VSEPR theory?
A) trigonal pyramidal
B) trigonal
C) tetrahedral
D) linear
E) bent
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following molecules does not have a dipole moment?
A) CS2
B) H2S
C) CH2Cl2
D) PH3
E) CH2O
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the nitrate ion (NO3-) , nitrogen and oxygen are held together by
A) ionic interactions.
B) covalent bonds.
C) dative bonds.
D) electronegativity.
E) network bonds.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Hydrazine, N2H4, is a good reducing agent that has been used as a component in rocket fuels. Select its Lewis structure.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) None of the above is correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to VSEPR theory, a molecule with the general formula AX5E will have a ______ molecular shape.
A) tetrahedral
B) trigonal bipyramidal
C) square pyramidal
D) octahedral
E) see-saw
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the correct Lewis structure for nitrogen trifluoride, NF3.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular shape of ClO3F as predicted by the VSEPR theory? 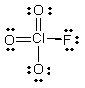
A) trigonal pyramidal
B) square planar
C) square pyramidal
D) tetrahedral
E) octahedral
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the ideal bond angles in AsCl3 using the molecular shape given by the VSEPR theory.
A) 90°
B) 109°
C) 120°
D) 180°
E) between 110 and 120°
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following molecules and ions will have a planar geometry?
A) PCl3
B) BF4-
C) XeF4
D) BrF5
E) H3O+
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the actual bond angle in SeCl2 using the VSEPR theory.
A) more than 120°
B) between 109° and 120°
C) between 90° and 109°
D) exactly 90°
E) less than 90°
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular shape of SiF62- as predicted by the VSEPR theory? 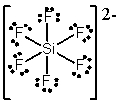
A) trigonal bipyramidal
B) hexagonal
C) tetrahedral
D) see-saw
E) octahedral
Correct Answer

verified
Correct Answer
verified
True/False
Boron never achieves an octet in any of its compounds.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electron pairs are shared between the carbon atoms in C2H4?
A) 5
B) 4
C) 3
D) 2
E) 1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which one of the following species is the best Lewis structure a resonance structure?
A) NH3
B) CO2
C) SF6
D) O2
E) CO32-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular shape of N2O as predicted by the VSEPR theory? 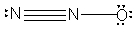
A) trigonal pyramidal
B) trigonal planar
C) angular
D) bent
E) linear
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 109
Related Exams