A) The reaction involves two steps and occurs fastest with primary alkyl halides.
B) The reaction involves one step and occurs fastest with primary alkyl halides.
C) The reaction involves one step and occurs fastest with tertiary alkyl halides.
D) The reaction involves two steps and occurs fastest with tertiary alkyl halides.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about the SN2 mechanism for nucleophilic substitution reactions is true?
A) Involves one step and occurs with retention of configuration.
B) Involves two steps and occurs with inversion of configuration.
C) Involves one step and occurs with inversion of configuration.
D) Involves one step and occurs with racemization.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following anions is the most nucleophilic in polar aprotic solvents?
A) F-
B) Cl-
C) Br-
D) I-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is not true?
A) Sodium ethoxide is a better nucleophile than sodium tert-butoxide.
B) Sodium tert-butoxide and sodium ethoxide have similar strengths as bases.
C) Sterically hindered bases are also called nonnucleophilic bases.
D) Steric hindrance decreases basicity but not nucleophilicity.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is not true?
A) In polar protic solvents, nucleophilicity decreases down a column of the periodic table as the size of the anion increases.
B) Nucleophilicity is affected by the solvent used in a substitution reaction.
C) Polar protic solvents are capable of intermolecular hydrogen bonding.
D) Polar protic solvents solvate both cations and anions.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name of the following compound? 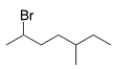
A) 2-Bromo-5-methyloctane
B) 2-Bromo-3-methylheptane
C) 2-Bromo-5-methylheptane
D) 6-Bromo-3-methylheptane
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the following reaction, use the identity of the alkyl halide and nucleophile to determine which substitution mechanism occurs. Then determine which solvent affords the faster reaction. 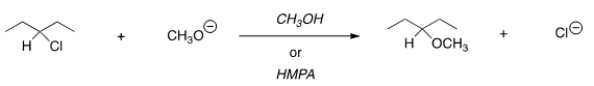
A) SN1, CH3OH
B) SN1, HMPA
C) SN2, CH3OH
D) SN2, HMPA
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the product of the nucleophilic substitution reaction shown below? 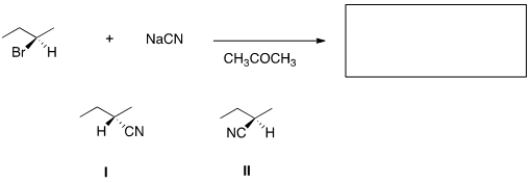
A) Only I
B) Only II
C) I and II
D) None
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about the Hammond postulate is true?
A) In an exothermic reaction, lowering the energy of the transition state increases the activation energy, Ea.
B) In an endothermic reaction, the more stable product forms faster.
C) In an endothermic reaction, the less stable product forms faster.
D) In an endothermic reaction, the activation energy, Ea, is similar for both products.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the following in order of decreasing leaving group ability, putting the best first.
A) III > II > IV > I
B) III > II > I > IV
C) IV > I > II > III
D) II > III > I > IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction of tert-butyl bromide, (CH3) 3CBr, with ethanol affords the substitution product tert-butyl ethyl ether, (CH3) 3COCH2CH3, in acidic conditions. What would happen to the rate of the reaction if the concentration of ethanol was doubled?
A) The rate remains the same.
B) The rate decreases by a factor of 2.
C) The rate increases by a factor of 2.
D) The rate increases by a factor of 4.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following alkyl halides is a vinyl alkyl halide? 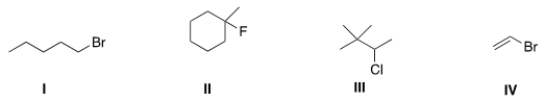
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the following ions in order of increasing nucleophlicity in polar protic solvents, starting with the least nucleophilic ion.
A) I < II < III < IV
B) IV < III < II < I
C) I < II < IV < III
D) IV < III < I < II
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name of the following compound? 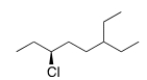
A) (R) -3-Chloro-6-ethyloctane
B) (S) -3-Chloro-6-ethyloctane
C) (S) -6-Chloro-3-ethyloctane
D) (R) -6-Chloro-3-ethyloctane
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the starting material in the reaction shown below? 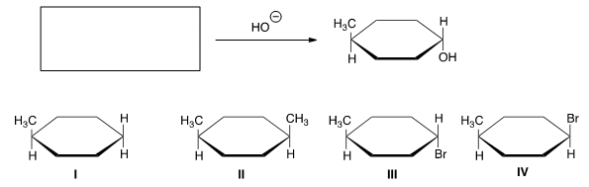
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about the reactions of alkyl halides is true?
A) The characteristic reactions of alkyl halides are addition and elimination.
B) The characteristic reactions of alkyl halides are addition and substitution.
C) The characteristic reactions of alkyl halides are elimination and substitution.
D) The characteristic reactions of alkyl halides are oxidation and reduction.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following carbocations is the most stable? 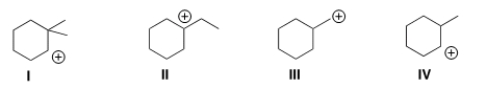
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name of the following compound? 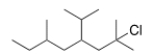
A) 2-Chloro-4-isopropyl-2,6-dimethyloctane
B) 2-Chloro-4-isopropyl-2,7-dimethylnonane
C) 2,6-Dimethyl-2-chloro-4-isopropyloctane
D) 7-Chloro-5-isopropyl-3,7-dimethyloctane
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the product of the nucleophilic substitution reaction shown below? 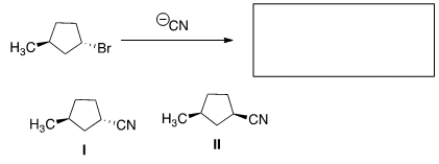
A) Only I
B) Only II
C) I and II
D) None
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name of the following compound? 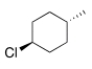
A) trans-4-Methylcyclohexyl chloride
B) trans-p-Chloromethylcyclohexane
C) trans-4-Methyl-1-chlorocyclohexane
D) trans-1-Chloro-4-methylcyclohexane
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 64
Related Exams