A) sublimation
B) condensation
C) effusion
D) diffusion
Correct Answer

verified
Correct Answer
verified
True/False
At room temperature,H2 molecules have the same average kinetic energy as O2.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The figure below is an example of which gas law? 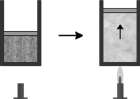
A) Charles'
B) Boyle's
C) Avagadro's
D) Dalton's
Correct Answer

verified
Correct Answer
verified
True/False
Charles's law can be stated mathematically as: P1V1 = P2V2
Correct Answer

verified
Correct Answer
verified
True/False
The vapor pressure of a liquid depends on the volume of the partially filled container.
Correct Answer

verified
Correct Answer
verified
True/False
The specific heat of ice is the amount of heat required to melt a specified weight to liquid water.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 3.2 liter sample of gas is at 40 C and 1.0 atmosphere of pressure.If the temperature decreases to 20 C and the pressure decreases to 0.60 atmospheres,what is the new volume in liters?
A) 5.7
B) 5.0
C) 1.8
D) 0.20
Correct Answer

verified
Correct Answer
verified
True/False
Polar liquids would normally have higher heats of vaporization than non-polar liquids.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Who developed the concept of partial pressure of gases in a mixture?
A) Charles
B) Boyle
C) Avogadro
D) Dalton
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The deposition of water vapor occurs when frost forms on a window.The correct equation to represent this phase change is _____ .
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the volume in liters of 3.50 moles of gas at STP.
A) 6.4
B) 44.8
C) 3.5
D) 78.4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The specific heat of ice is 0.48 cal/g C.How much heat will it take to raise the temperature of 10 g of ice from -30 C to -20 C?
A) 0.48 cal
B) 4.8 cal
C) 48 cal
D) 10 cal
Correct Answer

verified
Correct Answer
verified
True/False
There is no such thing as an ideal gas,although helium and nitrogen come close.
Correct Answer

verified
Correct Answer
verified
True/False
Cohesive forces are the attractive forces associated with potential energy.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When a liquid sample is taken from sea level to a higher elevation,what happens to the external (atmospheric) pressure on the liquid and the boiling point of the liquid?
A) both decrease
B) both increase
C) pressure goes down,but boiling point goes up
D) pressure goes up,but boiling point goes down
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using d = m/V,what is the volume (V) of a solid that has a density of 3.14g/cm3 and has a mass of 7.04 grams?
A) 22.1 cm3
B) 22.11 cm3
C) 2.24 cm3
D) 2.242 cm3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following gases is least likely to behave ideally?
A) He
B) N2
C) HCl
D) H2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the figure below which shows the compression phase of a piston in your car's engine.This is an example of which gas law? 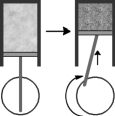
A) Charles'
B) Boyle's
C) Avagadro's
D) Dalton's
Correct Answer

verified
Correct Answer
verified
True/False
At STP,the vapor pressure of CH3CH2CH2CH2CH3 is less than CH3CH2CH2CH3.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Increasing the pressure on a gas at constant temperature does what to the volume of the gas?
A) increases
B) decreases
C) has no effect
D) increases up to a certain point
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 88
Related Exams