A) The gas molecules are large enough to occupy a substantial amount of space.
B) A large number of molecules have speeds greater than the average speed.
C) The gas molecules have a very low molar mass.
D) The gas molecules attract one another.
E) None of these.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of oxygen gas has a volume of 1.72 L at 27°C and 800.0 torr.How many oxygen molecules does it contain?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the equation: 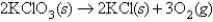 A 3.00-g sample of KClO3 is decomposed and the oxygen at 24.0°C and 0.717 atm is collected.What volume of oxygen gas will be collected assuming 100% yield?
A 3.00-g sample of KClO3 is decomposed and the oxygen at 24.0°C and 0.717 atm is collected.What volume of oxygen gas will be collected assuming 100% yield?
A) ![]() mL
mL
B) ![]() mL
mL
C) ![]() mL
mL
D) ![]() mL
mL
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At 1000°C and 10.torr,the density of a certain element in the gaseous state is 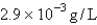 .The element is:
.The element is:
A) F
B) He
C) Na
D) Zn
E) Hg
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Four identical 1.0-L flasks contain the gases He,Cl2,CH4,and NH3,each at 0°C and 1 atm pressure. -For which gas do the molecules have the highest average velocity?
A) He
B) Cl2
C) CH4
D) NH3
E) all gases the same
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given a cylinder of fixed volume filled with 1 mol of argon gas,which of the following is correct? (Assume all gases obey the ideal gas law. )
A) If the temperature of the cylinder is changed from 25°C to 50°C,the pressure inside the cylinder will double.
B) If a second mole of argon is added to the cylinder,the ratio T/P would remain constant.
C) A cylinder of identical volume filled with the same pressure of helium must contain more atoms of gas because He has a smaller atomic radius than argon.
D) Two of the above.
E) None of the above.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Boyle's law states that:
A) Equal amounts of gases occupy the same volume at constant temperature and pressure.
B) The volume of a fixed amount of gas is inversely proportional to its pressure at constant temperature.
C) The volume of a fixed amount of gas is directly proportional to its temperature in Kelvin at constant pressure.
D) The total pressure of a mixture of gases is the simple sum of the partial pressure of all of the gaseous compounds.
E) The rates of effusion of gases are inversely proportional to the square roots of their molar masses.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following pollutant gases is not produced directly in a combustion engine?
A) CO
B) CO2
C) O3
D) NO
E) NO2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The local weather forecaster reports that the current barometric pressure is 30.4 inches of mercury.What is the current pressure in atmospheres?
A) 1.02 atm
B) 10.29 atm
C) 1.00 atm
D) 4.05 atm
E) 910 atm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not a postulate of the kinetic molecular theory?
A) Gas particles have most of their mass concentrated in the nucleus of the atom.
B) The moving particles undergo perfectly elastic collisions with the walls of the container.
C) The forces of attraction and repulsion between the particles are insignificant.
D) The average kinetic energy of the particles is directly proportional to the absolute temperature.
E) All of the above are postulates of the kinetic molecular theory.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 1.00-g sample of a gaseous compound of boron and hydrogen occupies 0.820 L at 1.00 atm and 3°C.What is the molecular formula for the compound?
A) BH3
B) B2H6
C) B4H10
D) B3H12
E) B5H14
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement is inconsistent with the kinetic theory of an ideal gas?
A) The forces of repulsion between gas molecules are very weak or negligible.
B) Most of the volume occupied by a gas is empty space.
C) When two gas molecules collide,they both gain kinetic energy.
D) The average kinetic energy of a gas is proportional to the absolute temperature.
E) Gas molecules move in a straight line between collisions.
Correct Answer

verified
Correct Answer
verified
Showing 121 - 132 of 132
Related Exams