A) 64.00 u
B) 63.55 u
C) 63.45 u
D) 64.31 u
E) 29.00 u
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following ratios of masses were obtained with a mass spectrometer: 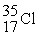 /
/ 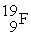 = 1.8406,
= 1.8406, 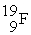 /
/ 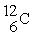 = 1.5832 What is the mass of a
= 1.5832 What is the mass of a 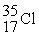 atom in atomic mass units?
atom in atomic mass units?
A) 35.45 u
B) 36.36 u
C) 13.95 u
D) 35.00 u
E) 34.97 u
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many atoms of rubidium-85 are in 87.2 g of rubidium? Rubidium-85 is 72.2 % abundant.
A) 5.16 × 1046 atoms
B) 4.44 × 1023 atoms
C) 8.51 × 1023 atoms
D) 6.14 × 1022 atoms
E) 1.02 × 1024 atoms
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many atoms of hydrogen are present in 1.5 lb of hydrogen peroxide, which is 5.93% hydrogen? (1 lb = 454 grams)
A) 2.4 × 1025 atoms
B) 1.21 × 1025 atoms
C) 1.2 × 1025 atoms
D) 2.41 × 1025 atoms
E) 1.2 × 1020 atoms
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When decomposed chemically, 73.0 grams of a sample of HCl produce 71.0 g of Cl2 and 2.0 g of H2, while 34.0 g of a sample of H2S produce 32.0 g of S and 2.0 g of H2. This is an example of the Law:
A) of Conservation of Mass
B) of Multiple Proportions
C) of Definite Proportions
D) E = mc2
E) of Simple Whole Numbers
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a correct feature of the nuclear atom proposed by Rutherford?
A) All atoms of an element have the same mass.
B) The atom is mostly empty space.
C) The number of neutrons and electrons in the atom are equal.
D) The majority of alpha particles to strike the foil "bounced back."
E) It is like the "plum pudding" model.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the INCORRECT statement from those given below.
A) Atoms retain their identity during a chemical reaction.
B) All matter is composed of atoms.
C) Atoms combine in small, whole-numbered ratios.
D) All atoms of a given element are identical.
E) Different ratios of atoms produce different compounds.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Ernest Rutherford is credited with: I) the nuclear model of the atom II) the identification of alpha and beta particles III) the discovery of protons IV) the prediction of a third, neutral, subatomic particle
A) I and II
B) I and III
C) I, II, III
D) II, III, IV
E) I, II, III, IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A certain element contains eleven atoms of mass 95.952 u for every four atoms of mass 98.949 u. Compute the average atomic weight of this element.
A) 97.451 u
B) 96.754 u
C) 98.150 u
D) 105.16 u
E) 96.952 u
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Lead-204 has a relative abundance of 1.48%. What size block of lead (in cubic centimeters) will contain 8.34 × 1021 atoms of lead-204? The density of lead is 11.35 g/cm3.
A) 3.75 × 10-3
B) 0.253
C) 194
D) 17.1
E) 16.8
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement below is true?
A) Metals gain electrons to have a positive charge.
B) Metals gain electrons to have a negative charge.
C) Metals lose electrons to have a positive charge.
D) Nonmetals lose electrons.
E) Transition metals can gain 2 or more electrons to become metal ions.
Correct Answer

verified
Correct Answer
verified
True/False
Robert Millikan determined the charge on an electron.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the isotopic atomic mass of an isotope if 9.7023 × 1022 atoms weighs 4.0256 g?
A) 0.64858
B) 24.305
C) 24.986
D) 13.728
E) 4.0256
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An isotope with mass number 81 has eleven more neutrons than protons. This is an isotope of what element?
A) Tl
B) Yb
C) Zr
D) Br
E) Nb
Correct Answer

verified
Correct Answer
verified
True/False
All atoms of a given element are identical.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following would be unaffected by an electric field?
A) alpha particles
B) beta particles
C) gamma rays
D) protons
E) electrons
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many protons, neutrons, and electrons are in 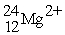 ?
?
A) 12 protons, 10 electrons, 12 neutrons
B) 12 protons, 12 electrons, 12 neutrons
C) 12 protons, 12 electrons, 24 neutrons
D) 24 protons, 10 electrons, 12 neutrons
E) 10 protons, 12 electrons, 24 neutrons
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is true concerning the masses of individual Cl atoms?
A) All atoms have a mass of 35.45 u.
B) Most of the atoms have a mass of 35.45 u.
C) Some of the atoms have a mass of 35.45 u.
D) None of the atoms have a mass of 35.45 u.
E) All atoms have a mass of 17 u.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Dalton's atomic theory is based on several assumptions, which are listed below. Which of these assumptions is strictly correct? I) All atoms of the same element are identical. II) Atoms are indivisible and unchangeable. III) Chemical changes are the result of the combination, separation, and rearrangement of atoms.
A) I, II, and III are correct.
B) I and III are correct.
C) II and III are correct.
D) I and II are correct.
E) III is correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many Cu atoms are present in a 75.0 cm length of 20-gauge copper wire? A 20-gauge wire has a diameter of 0.03196 in. Copper's density is 8.92 g/cm3.
A) 1.31 × 1021
B) 3.28 × 1022
C) 8.08 × 1021
D) 1.04 × 1022
E) 2.08 × 1022
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 100
Related Exams