A) N = λR
B) R = λN
C) R = λ/N
D) R = N/ λ
E) R = λ ln N
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The half-life of a radioactive substance is:
A) half the time it takes for the entire substance to decay
B) usually about 50 years
C) the time for radium to change into lead
D) calculated from E = mc2
E) the time for half the substance to decay
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A nucleus with mass number A and atomic number Z undergoes - decay. The mass number and atomic number, respectively, of the daughter nucleus are:
A) A, Z - 1
B) A - 1, Z
C) A + 1, Z - 1
D) A, Z + 1
E) A, Z
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In an alpha decay the disintegration energy appears as:
A) photon energies
B) the kinetic energies of the alpha and the daughter nucleus
C) the excitation energy of the daughter nucleus
D) the excitation energy of the alpha particle
E) heat
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Magnesium has atomic number 12, hydrogen has atomic number 1, and helium has atomic number 2. In the nuclear reaction 24Mg + 2H ( ) + 4He the missing quantity is:
A) 23Na (Z = 11)
B) 22Ne (Z = 10)
C) 21Na (Z = 11)
D) 21Ne (Z = 10)
E) 22Na (Z = 11)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The smallest particle of any chemical element that can exist by itself and yet retain the qualities that distinguish it as that element is:
A) an electron
B) a proton
C) a neutron
D) an atom
E) a molecule
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Iron has atomic number 26. Naturally mined iron contains isotopes of mass numbers 54, 56, 57, and 58. Which of the following statements is FALSE?
A) every atom of iron has 26 protons
B) some iron atoms have 30 neutrons
C) some iron atoms have 54 neutrons
D) the isotopes may be separated in a mass spectrometer
E) there are four kinds of naturally occurring iron atoms with the same chemical properties
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A radioactive atom X emits a - particle. The resulting atom:
A) must be very reactive chemically
B) has an atomic number that is one more than that of X
C) has a mass number that is one less than that of X
D) has an atomic number that is one less than that of X
E) is the same chemical element as X
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Radioactive element A decays to the stable element B with a half-life T. Starting with a sample of pure A and no B, which graph below most correctly shows the number of A atoms, NA, as a function of time t? 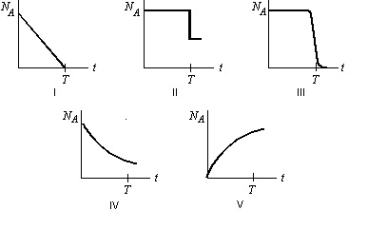
A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following nuclides is least stable?
A) 52Fe (Z = 26)
B) 115Nd (Z = 60)
C) 175Lu (Z = 71)
D) 208Pb (Z = 82)
E) 238U (Z = 92)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Bromine, with atomic mass 79.942 u, is composed of nearly equal amounts of two isotopes, one of which contains 79 nucleons per atom. The mass number of the other isotope is:
A) 78
B) 79
C) 80
D) 81
E) 82
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A proton in a large nucleus:
A) has a net attractive force on all other protons in the nucleus
B) has a net repulsive force on all other protons in the nucleus
C) has a net repulsive force on all other neutrons in the nucleus
D) has a net attractive force on some protons in the nucleus and a net repulsive force on others
E) has a net attractive force on some neutrons in the nucleus and a net repulsive force on others
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Starting with a sample of pure 66Cu, 7/8 of it decays into Zn in 15 minutes. The corresponding half-life is:
A) 3.75 minutes
B) 5 minutes
C) 7 minutes
D) 10 minutes
E) 15 minutes
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The half-life of a radioactive isotope is 6.5 h. If there are initially 48 * 1032 atoms of this isotope, the number of atoms of this isotope remaining after 26 h is:
A) 12 * 1032
B) 6 * 1032
C) 3 * 1032
D) 6 *104
E) 3 * 102
Correct Answer

verified
Correct Answer
verified
Multiple Choice
One curie is equivalent to:
A) one Becquerel
B) one decay per second
C) 3.0 x 108 decays per second
D) 3.7 x 1010 decays per second
E) 106 Becquerel
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The model of the nucleus in which each nucleon has its own well-defined quantum numbers is called the:
A) collective model
B) quantum model
C) liquid drop model
D) independent model
E) atomic model
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The 66Cu (Z = 29) produced in a nuclear bombardment is unstable, changing to 66Zn (Z = 30) by the emission of:
A) a proton
B) a gamma ray photon
C) a positron
D) an electron
E) an alpha particle
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The half-life of a radioactive isotope is 140 days. In how many days does the decay rate of a sample of this isotope decrease to one fourth its initial decay rate?
A) 35 days
B) 70 days
C) 105 days
D) 210 days
E) 280 days
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A certain nucleus, after absorbing a neutron, emits a - and then splits into two alpha particles. The (A, Z) of the original nucleus must have been:
A) 6, 2
B) 6, 3
C) 7, 2
D) 7, 3
E) 8, 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An alpha particle is:
A) a helium atom with two electrons removed
B) an aggregate of two or more electrons
C) a hydrogen atom
D) the ultimate unit of positive charge
E) sometimes negatively charged
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 68
Related Exams