Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is (are) a keto-enol tautomeric pair(s) ? 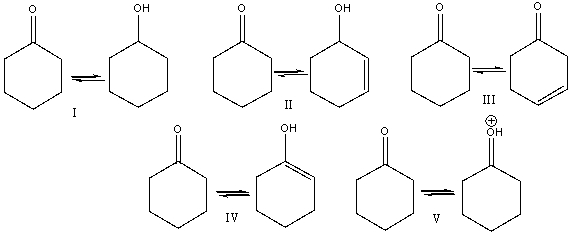
A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Essay
Provide both resonance structures of the enolate formed when the following ketone is treated with a base. 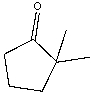
Correct Answer

verified
Correct Answer
verified
Essay
Provide the reactants that would give the following aldol condensation product. 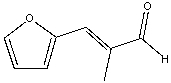
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the product(s) for the following reaction. 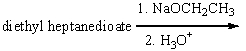
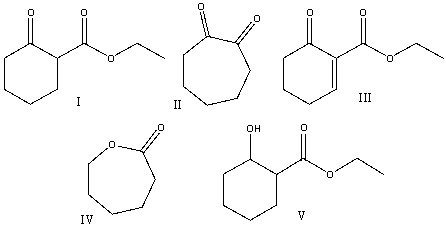
A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Essay
Provide the reactant(s) that will yield the following Claisen condensation product. 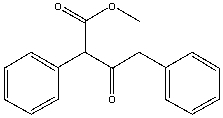
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the major product for the following reaction. 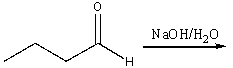
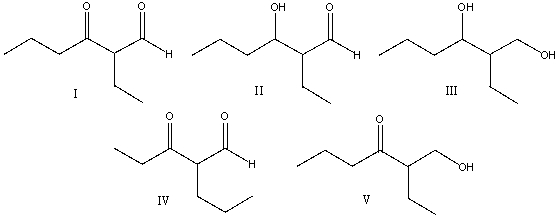
A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Provide the reactants necessary to prepare the following compound using Robinson annulation. 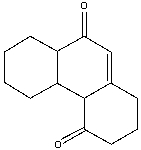
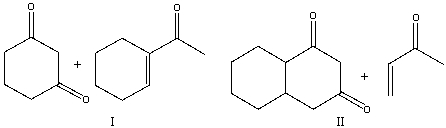
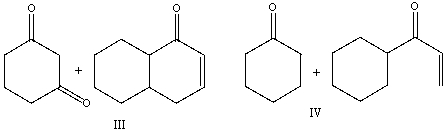
A) I
B) II
C) III
D) IV
E) I & III
Correct Answer

verified
Correct Answer
verified
Essay
Provide the reactant(s) that would give the following possible aldol condensation product. 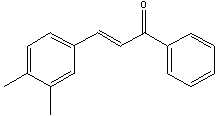
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the product for the following reaction. 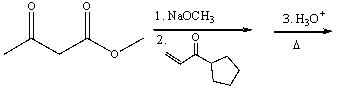
A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the most acidic hydrogen in the following compound? 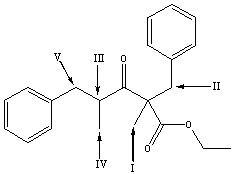
A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
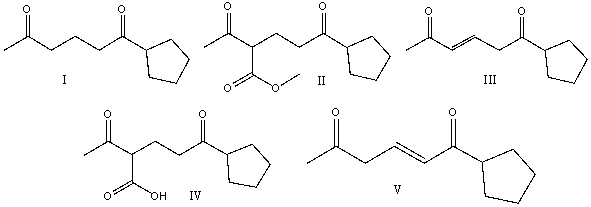
Multiple Choice
Predict the product for the following reaction. 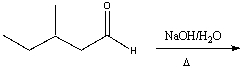
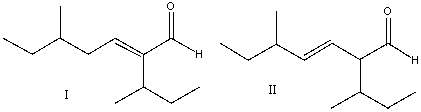
A) I
B) II
C) III
D) IV
E) none of these
Correct Answer

verified
Correct Answer
verified
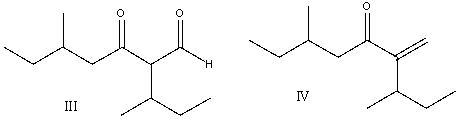
Multiple Choice
Provide the reactants that would give the following aldol condensation product. 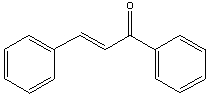
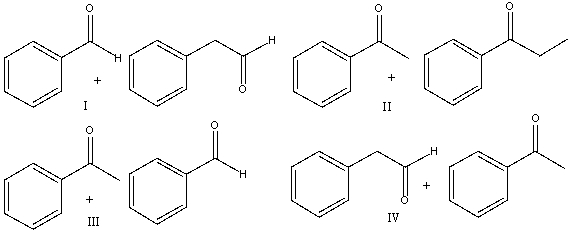
A) I
B) II
C) III
D) IV
E) none of these
Correct Answer

verified
Correct Answer
verified
Essay
Using 1-propanol as your only source of carbon and using any other reagents of your choice provide a stepwise synthesis for the following conversion. 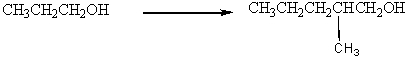
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds would undergo racemization in presence of a base? 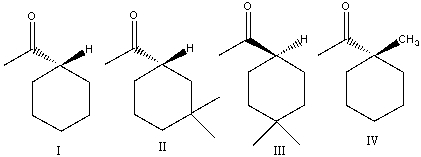
A) I
B) II
C) III
D) IV
E) II & III
Correct Answer

verified
Correct Answer
verified
Essay
Provide the reagents necessary to carry out the following conversion using a malonic ester synthesis. 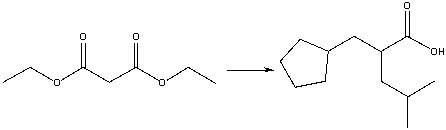
Correct Answer

verified
1. NaOCH2CH3
2. (CH3)2CH...View Answer
Show Answer
Correct Answer
verified
2. (CH3)2CH...
View Answer
Multiple Choice
Predict the product(s) for the following reaction. 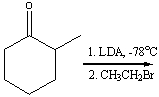
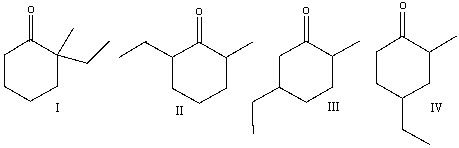
A) I
B) II
C) III
D) IV
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the product(s) for the following reaction. 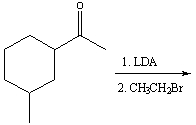
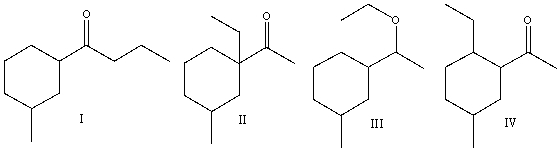
A) I
B) II
C) III
D) IV
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is (are) a keto-enol tautomeric pair(s) ? 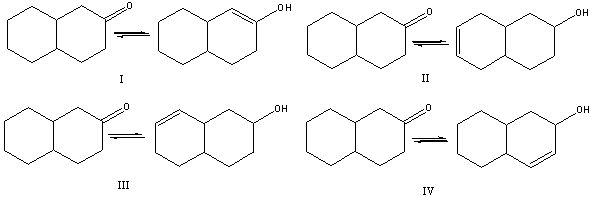
A) I
B) II
C) III
D) IV
E) None of these
Correct Answer

verified
Correct Answer
verified
Essay
Provide the structure of the enol when 3,3,6-trimethyl-4-heptanone is treated with acid.
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 131
Related Exams