A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the following reaction, identify the Lewis base. 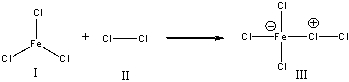
A) I
B) II
C) III
D) None of these
Correct Answer

verified
Correct Answer
verified
Essay
Which of the following compounds is more acidic? Explain why. 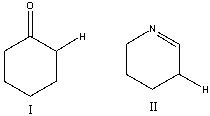
Correct Answer

verified
Compound I is more acidic. The conjugate...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
For the following reaction identify the acid and the base and predict the products. Draw the curved arrow mechanism for the formation of products and predict the direction of the equilibrium. 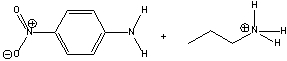
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the following reaction, identify the Lewis base. 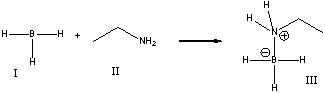
A) I
B) II
C) III
D) None of these
Correct Answer

verified
Correct Answer
verified
Essay
Predict the products for the following acid-base reaction. 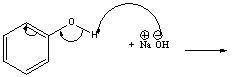
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the following acid-base reaction, predict which side the equilibrium is favored. 
A) favor right side
B) favor left side
C) neither
Correct Answer

verified
Correct Answer
verified
Essay
For the following reaction label the acid, base, conjugate acid and conjugate base. 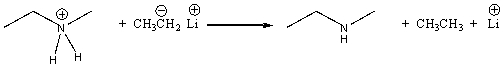
Correct Answer

verified
Correct Answer
verified
Essay
Provide a curved arrow mechanism for the following acid-base reaction. 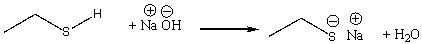
Correct Answer

verified
Correct Answer
verified
Essay
For the following reaction label the acid, base, conjugate acid and conjugate base. 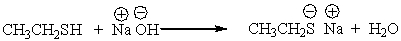
Correct Answer

verified
Correct Answer
verified
Short Answer
In a Brønsted-Lowry acid-base reaction the product(s) is (are) a _______.
Correct Answer

verified
conjugate ...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
For the following acid-base reaction, predict which side the equilibrium is favored. Explain why. 
Correct Answer

verified
Favors the right side.
Both the base and...View Answer
Show Answer
Correct Answer
verified
Both the base and...
View Answer
Multiple Choice
What is the conjugate base of CH3CH2SH?
A) CH3CH2S-
B) CH3CH2-
C) CH3CH2SH2+
D) CH3CH2S2-
E) None of these
Correct Answer

verified
Correct Answer
verified
Essay
Determine if CH3CH2ONa is a suitable reagent to deprotonate the following compound. Explain why. Draw the complete reaction, including the curved arrow mechanism. 
Correct Answer

verified
No.  The base has negative cha...
The base has negative cha...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Which of the following compounds is more acidic? Explain why. HF and HBr
Correct Answer

verified
HBr is more acidic than HF. The Br- ion h...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Determine if H2O is a suitable reagent to protonate the following compound. 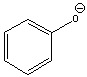
A) yes
B) no
Correct Answer

verified
Correct Answer
verified
Essay
Determine if NaOH is a suitable reagent to deprotonate the following compound. Explain why. 
Correct Answer

verified
No.
The base has negative char...View Answer
Show Answer
Correct Answer
verified
The base has negative char...
View Answer
Essay
For the following reaction, identify the Lewis acid and the Lewis base. 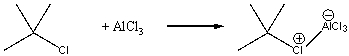
Correct Answer

verified
Correct Answer
verified
Essay
Predict the product(s) for the following reaction and draw the curved arrow mechanism. 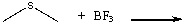
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the indicated protons in decreasing order (most to least) of acidity. 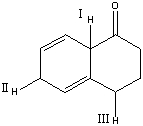
A) II>I>III
B) II>III>I
C) I>III>II
D) I>II>III
E) III>II>I
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 126
Related Exams